

| ʵ�鲽�� | ���� | ���� |
��SO2����ͨ�� NaHCO3��Na2CO3��Һ�� ����KMnO4��Һ�� Ʒ����Һ�� ����ʯ��ˮ ��SO2����ͨ�� NaHCO3��Na2CO3��Һ�� ����KMnO4��Һ�� Ʒ����Һ�� ����ʯ��ˮ |
NaHCO3��Na2CO3��Һ�����ݣ� Ʒ����Һ����ɫ�� ����ʯ��ˮ����� NaHCO3��Na2CO3��Һ�����ݣ� Ʒ����Һ����ɫ�� ����ʯ��ˮ����� |
H2SO3����ǿ��H2CO3 |
| ʵ�鲽�� | ���� | ���� |
| ��SO2����ͨ�� NaHCO3��Na2CO3��Һ�� ����KMnO4��Һ�� Ʒ����Һ�� ����ʯ��ˮ |
NaHCO3��Na2CO3��Һ�����ݣ� Ʒ����Һ����ɫ�� ����ʯ��ˮ����� |
H2SO3����ǿ��H2CO3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ʼ����ʱ��pH | ������ȫʱ��pH | |
| Mg2+ | 9.6 | 11.0 |
| Ca2+ | 12.2 | c��OH-��=1.8mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�߿����� ���ͣ��ƶ���






�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
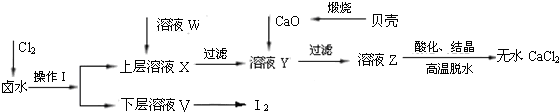
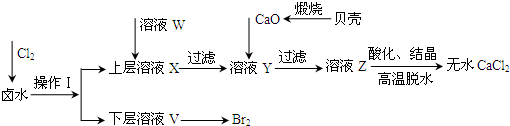
ʵ�����Ժ���Ca2+��Mg2+��Cl����SO42����Br�������ӵ�±ˮΪ��Ҫԭ���Ʊ���ˮCaCl2��Br2���������£�

��1��������ʹ�õ��Լ���______�����õ���Ҫ����������_______��
��2��������ҺW��Ŀ����______����CaO������ҺY��pH�����Գ�ȥMg2+���ữ��ҺZʱ��ʹ�õ��Լ�Ϊ_____��
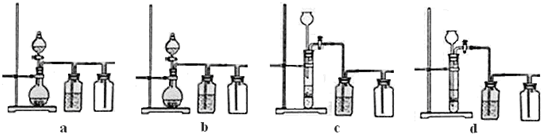
��3��ʵ�����ñ�����ϡ���ᷴӦ�Ʊ����ռ����壬����װ���к�������______��

��4����ҵ��ģ��ˮ���峣�ÿ������������崵����������ʹ�����������ռ�SO2���������Դﵽ������Ŀ�ģ���д���÷�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����һ�и�һ���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������


| ʵ�鲽�� | ���� | ���� |
______ | ______ | H2SO3����ǿ��H2CO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com