
| ��� | I | II | III |
| �������/ml | 100 | 100 | 100 |
| A������/g | 1.90 | 3.42 | 3.80 |
| ��������/ml | 448 | 716.8 | 672 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ������ѧ����һ�ּ�⻯ѧ�Ծ����������� ���ͣ���ѡ��
��һ������CO2������ͨ��500ml NaOH��Һ�У���ַ�Ӧ����Һ�����������õ������ᾧˮ�İ�ɫ����A 9.50g��ȡ3��������ͬ��A���ֱ���100ml��ͬ���ʵ���Ũ�ȵ����ᷴӦ���õ��������������״������A��������ϵ���±���ʾ���� ��
| ��� | I | II | III |
| �������/ml | 100 | 100 | 100 |
| A������/g | 1.90 | 3.42 | 3.80 |
| ��������/ml | 448 | 716.8 | 672 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�Ƹ��и�����ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijʵ��С��Ϊ�о��������ȡ�Ͳ�������ʣ���������ʵ�顣
ʵ��I:�Ʊ�����
ʵ������������������ˮ��Һ�Ʊ������װ����ͼ��ʾ(���ȡ�����������̶�װ�þ�����ȥ����ʵ��������£�
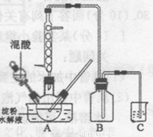
�ٽ�һ�����ĵ���ˮ��Һ��������ƿ��
�ڿ��Ʒ�ӦҺ�¶���55〜600C�����£��߽�������μ�һ�����������������Ļ��� (65%HNO3��98%H2S04��������Ϊ2 :1.5)��Һ
�۷�Ӧ3h���ң���ȴ�����˺����ؽᾧ�ò��ᾧ�塣
������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6��12HNO3��3H2C2O4��9NO2����3NO����9H2O
C6H12O6��8HNO3��6CO2����8NO����10H2O
3 H2C2O4��2HNO3��6CO2����2NO����4H2O
(1)��������Ƿ�ˮ����ȫ�����õ��Լ�Ϊ________��
(2)ʵ����������μӹ��죬�����²�������½�����ԭ����________��
ʵ��II �����ᾧ���нᾧˮ�ⶨ
���ᾧ��Ļ�ѧʽ�ɱ�ʾΪH2C2O4 • xH2O,Ϊ�ⶨx��ֵ,��������ʵ�飺
�ٳ�ȡ6.3gij���ᾧ�����100.0mL��ˮ��Һ��
��ȡ25.00mL������Һ������ƿ�У���������ϡH2SO4����Ũ��Ϊ0. 5mol/L��KMnO4��Һ�ζ����ζ��յ�ʱ����KMnO4 �����Ϊ10.00mL���ش�����������
��3��д��������Ӧ�����ӷ���ʽ________________��
��4������x=________��
��5���ζ�ʱ�������ַ�Ӧ���ʿ�ʼ�����������ӿ죬���ܵ�ԭ����________��
ʵ��III:����ȶ���
�������ϣ����ᾧ��(H2C2O4 •xH20),1000C��ʼʧˮ��100.5�����ҷֽ����H2O��CO��CO2��������ͼ���ṩ���������Լ������һ��ʵ�飬֤�����ᾧ��ֽ�õ��Ļ��������H2O��CO��CO2 (����װ�ú͵��ܵ���ͼ����ȥ������װ�ÿ��ظ�ʹ�ã���
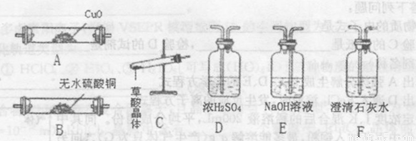
�ش��������⣺
(6)����װ�ð�����˳��Ϊ________��
(7)����B����ˮ����ͭ������________��
��8����֤��������к���CO��ʵ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ����һ�ּ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ������CO2������ͨ��500ml NaOH��Һ�У���ַ�Ӧ����Һ�����������õ������ᾧˮ�İ�ɫ����A 9.50g��ȡ3��������ͬ��A���ֱ���100ml��ͬ���ʵ���Ũ�ȵ����ᷴӦ���õ��������������״������A��������ϵ���±���ʾ���� ��
|
��� |
I |
II |
III |
|
�������/ml |
100 |
100 |
100 |
|
A������/g |
1.90 |
3.42 |
3.80 |
|
��������/ml |
448 |
716.8 |
672 |
����˵������ȷ����
A����I����Ʒ������ʣ��
B��������0.38g��Ʒ�������շų�����67.2mL
C��ԭNaOH�����ʵ���Ũ����0.3mol��L��1
D����������ʵ���Ũ�ȵ�0.5mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�����^����������ѧ�Ծ��������棩 ���ͣ������
��8�֣���1����֪20oCʱ���ܽ�ȣ�Na2CO3��S��21.2g�� NaHCO3��S��9.6g����20oC������������121.2g����̼������Һ��ͨ��������CO2���壬�����Ͽ�������NaHCO3__________g��С�������1λ��
��2����500mL KOH��Һ�л���ͨ��һ������CO2���壬��ַ�Ӧ���ڼ�ѹ������������Һ���õ���ɫ���塣��ͨ��CO2����Ϊ2.24L(��״����)���õ�11.9g�İ�ɫ���塣�����õ�KOH��Һ�����ʵ���Ũ��Ϊ______mol/L��
��3����һ���������Ƽ��뵽89gˮ�У���ȫ��Ӧ�����ҺΪ100g�������Һ����������Ϊ________��
��4�����мס�����ƿ��ɫ��Һ����֪���ǿ�����AlCl3��Һ��NaOH��Һ.��������ʵ�飺
��ȡ440ml����120ml�ҷ�Ӧ������1.56g������
��ȡ440ml����120ml��Ӧ��Ҳ����1.56g������
��ȡ120ml����Һ��400ml����Һ��Ӧ�������3.12g������ͨ����Ҫ�ļ���������ж���
����ҺΪ ��Һ������ҺΪ ��Һ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com