
��12�֣�NaCl��һ�ֻ���ԭ�ϣ������Ʊ�һϵ�����ʣ���Ӧ���������ַ�Ӧ��������ȥ�������ǵ�ת����ϵ��ͼ��ʾ����ش��������⣺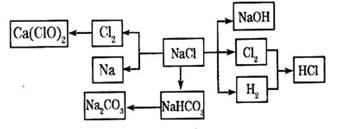
��1����ҵ�ϳ��õ������NaCl�ķ�����ȡ�����ƣ�NaCl�ۻ�ʱ�ƻ����Ӽ��Ĺ�������___________��������仯����ѧ�仯������
��2��д����ҵ����ȡHCl�Ļ�ѧ����ʽ________________________________________��
��3��д����ҵ����ȡ�ռ�����ӷ���ʽ________________________________________��
��4������ƴ���Ĺ�������Ϊ��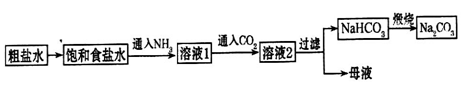
��NaHCO3��ȡNa2CO3�Ļ�ѧ����ʽ________________________________________��
��������������ʳ��ˮ��ͨ��NH3����ͨ��CO2����Ŀ���ǣ�____________________
___________________________________________________________________________��
��5���������һʵ��֤��Na2CO3��Һ���Ա�NaHCO3��Һ�ļ���ǿ��_________________
____________________________________________________________________________
��12�֣�ÿ��2�֣�
��1�������仯
(2)H2��Cl2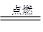 2HCl
2HCl
(3)2Cl����2H2O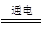 2OH����H2����Cl2��
2OH����H2����Cl2��
(4)2NaHCO3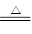 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��ΪNH3��������ˮ����Һ�������ԣ���ͨ��CO2ʹ֮�����ת��ΪHCO3-
��5������ 0.1mol��L��1��������ʵ���Ũ�ȣ���������Һ������pH��Na2CO3��ҺpH����NaHCO3��Һ��˵��Na2CO3��Һ���Ա�NaHCO3��Һ�ļ���ǿ�������������𰸣�
����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2010��2011ѧ��߶���ѧ����ĩͳ����ѧ����(ѡ��) ���ͣ�022
����һ����;�㷺�Ļ���ԭ�ϣ�ͬʱҲ��һ�����ص���Ⱦ������۸�����ǿ�ҵ��°����ã���ҵ��Ϊ�˴�����Cr2O![]() �����Է�ˮ��������ķ�������������ҵ��ˮ�м���������NaCl������ȣ�����Ϊ�缫���е�⣬�Ӷ�ʹ��Һ��pH��������ˮ������ת��Ϊ���ԣ�����һ��ʱ����Cr(OH)3��Fe(OH)3�������ɣ����˻��ճ�������ҵ��ˮ�и��ĺ����������ŷű���
�����Է�ˮ��������ķ�������������ҵ��ˮ�м���������NaCl������ȣ�����Ϊ�缫���е�⣬�Ӷ�ʹ��Һ��pH��������ˮ������ת��Ϊ���ԣ�����һ��ʱ����Cr(OH)3��Fe(OH)3�������ɣ����˻��ճ�������ҵ��ˮ�и��ĺ����������ŷű���
�Իش�
(1)����������NaCl����________
(2)�缫��Ӧʽ������________������________
(3)��Cr2O![]() ��ΪCr3ʮ�����ӷ���ʽΪ________
��ΪCr3ʮ�����ӷ���ʽΪ________
(4)��ҵ��ˮ�����Ա���Ե�ԭ��Ϊ________
(5)�ܷ����ʯī��ͭ�缫________(��ܡ����ܡ�)��ԭ��Ϊ________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com