
˵������Ħ����������ܺ��Ӱ����ӵ����ɣ�Ϊ�˲ⶨij����(���飬���������������̬��)��Ʒ��ƽ����Է���������ijѧ�����������ʵ�飺
��ȡһ���ྻ�����ﲢ���к��ʽ�������ƿ��ȷ�������õ�����m1��
������ƿ��ͨ�����ĸ�������Ʒ�����ý�����ȷ�������ظ�������ֱ��ǰ�����γ����Ľ��������ͬ���õ�����m2��
������ƿ�ڼ���ˮ�����ý����������õ�����m3��
[�¶ȣ�T(K)��ѹǿ��p(kPa)��ˮ���ܶȣ���ˮ(g/L)��������ƽ��ʽ����29.0���������ܶȦ�����(g/L)]
�������ݸ�ͬѧ�IJ����ش��������⣺
(1)��������ظ�������ԭ����________��
(2)�����ڴ�ʵ���У��������ÿ�β�����������ͬ������½��У�________��
(3)��ƿ�ڿ���������(m����)��________(�г���ʽ����ͬ)��
(4)ƿ����Ʒ��������(m��Ʒ)��________��
(5)��ʵ������Ʒ����ƽ����Է���������________��
(6)ͨ����ʵ�飬��ó��Ľ�����________(дһ������)��
 �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
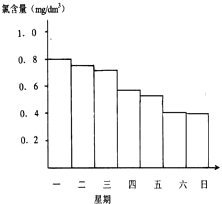 ��Ӿ��ˮ�ĺ�����Ӧ�ÿ�����0.5mg/L��1.0mg/L֮�䣬
��Ӿ��ˮ�ĺ�����Ӧ�ÿ�����0.5mg/L��1.0mg/L֮�䣬�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��p��qΪֱ����Դ������A��+2�۽�������X�Ƴɣ�B��C��DΪ���缫����ͨ��Դ������X������B����ͬʱC��D�������ݡ��Իش�

��1��pΪ ����A�������� ��Ӧ���Ӧ���ͣ���
��2��CΪ �����Թ����ռ��� ��DΪ �����Թ����ռ��� ��
��3��C���ĵ缫����ʽ�� ��
��4���ڵ������У����C��D�����ϲ����������ʵ���������£�
| ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| �����������������cm3�� | 6 | 12 | 20 | 29 | 39 | 49 | 59 | 69 | 79 | 89 |
| �����������������cm3�� | 2 | 4 | 7 | 11 | 16 | 21 | 26 | 31 | 36 | 41 |
��ϸ�����ϱ�����˵���õ�����ʵ�����ݿ��ܵ�ԭ���� ��
��5������Ӧ����һ��ʱ���A��B�缫������Һ��pH �����������С�����䡱����
��6������·��ͨ��0.004mol����ʱ��B�缫�ϳ�������X������Ϊ0.128g����˽�����Ħ������Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com