


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
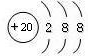

| C | 2- 2 |
| O | 2+ 2 |
| O | 2+ 2 |
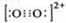
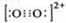
| O | 2+ 2 |
| C | 2- 2 |
| C | 2- 2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| C | 2- 2 |
| O | 2+ 2 |
| O | 2+ 2 |
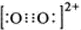
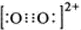
| O | 2+ 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ȷ���������ʵ���Ҫ���������ʽṹ����ش��������⣮
ȷ���������ʵ���Ҫ���������ʽṹ����ش��������⣮| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
| ���Ӿ��� | KCl | MgO | CaO |
| ������/kJ?mol-1 | 715 | 3791 | 3401 |
| C | 2- 2 |
| O | 2+ 2 |
| O | 2+ 2 |


| O | 2+ 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 [��ѧѡ��2-��ѧ�뼼��]�ҹ��ȼҵѸ�ٷ�չ��������Ծ��������λ������ֲ�˾�ϸ��������Ʒ������
[��ѧѡ��2-��ѧ�뼼��]�ҹ��ȼҵѸ�ٷ�չ��������Ծ��������λ������ֲ�˾�ϸ��������Ʒ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
| ���Ӿ��� | KCl | MgO | CaO |
| ������/kJ?mol-1 | 715 | 3791 | 3401 |
| C | 2-2 |
| O | 2+2 |
| O | 2+2 |
| O | 2+2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com