
�������������ѧ�γ����Ķ���ǿ�ᣮ��Ͷ��������ͭ�ķ�Ӧ������ش��������⣺
(1)����100 mL��18 mol��L��1��Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ�������������ڱ�״���µ����������________��
A��40.32 L
B��30.24 L
C��20.16 L
D��6.72 L
����ʹ������Ӧ����ʣ���ͭƬ�����ܽ⣬�������м��������ƣ�д����Ӧ�����ӷ���ʽ________��
(2)��������ͭ����һ����Ũ���ᷴӦ������Ӧ��ȫֹͣʱ�����ռ���������1.12 L(��״��)���������ijɷ���________����Ӧ�������ĵ���������ʵ�������Ϊ________��
A��0.1 mol
B��0.15 mol
C��0.2 mol
D��0.25 mol

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
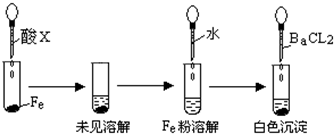
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

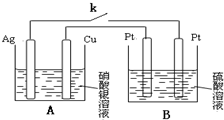
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2008?��ɽһģ�����ᡢ�������������ѧ�γ����ġ������ᡱ����͡������ᡱ�����ͭ��Ӧ��������ش��������⣺
��2008?��ɽһģ�����ᡢ�������������ѧ�γ����ġ������ᡱ����͡������ᡱ�����ͭ��Ӧ��������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com