
| ʵ����� | ʵ������� Ԥ��ʵ������ |
������� �������ӷ���ʽ��ʾ�� |
���� | |
| ����� | ����һ�� |
��ҺpH=8 | / | Na2S2O3��Һ�����ԣ������ᷴӦ�����л�ԭ�ԣ���Na2SO4�Ļ�ѧ���ʲ����ƣ� |
| �������� |
S2O32-+2H+=S��+SO2��+H2O | |||
| ����� | �������� |
| ʵ����� | ʵ������� Ԥ��ʵ������ |
������� �������ӷ���ʽ��ʾ�� |
���� | |
| ����� | ����һ���ò�����պȡNa2S2O3��Һ����ε�pH��ֽ�����룬����ֽ���ֵ���ɫ�����ɫ������ | |||
| ���������õι�ȡ����Na2S2O3��Һ���Թܣ�Ȼ����ε���3 mol?L-1H2SO4���� | �е���ɫ��������ɫ�̼�����ζ������� | |||
| ����� | ���������õι�ȡ����������ˮ���Թܣ�Ȼ����εμ�����Na2S2O3��Һ���� | ��ˮ��ɫ��dz | S2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+ |
| ʵ����� | ʵ������� Ԥ��ʵ������ |
������� �������ӷ���ʽ��ʾ�� |
���� | |
| ����� | ����һ���ò�����պȡNa2S2O3��Һ����ε�pH��ֽ�����룬����ֽ���ֵ���ɫ�����ɫ������ | |||
| ���������õι�ȡ����Na2S2O3��Һ���Թܣ�Ȼ����ε���3 mol?L-1H2SO4���� | �е���ɫ��������ɫ�̼�����ζ������� | |||
| ����� | ���������õι�ȡ����������ˮ���Թܣ�Ȼ����εμ�����Na2S2O3��Һ���� | ��ˮ��ɫ��dz | S2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鷽�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
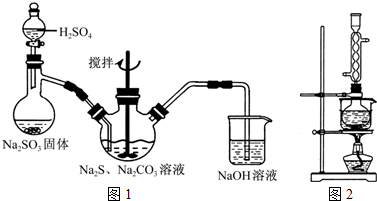
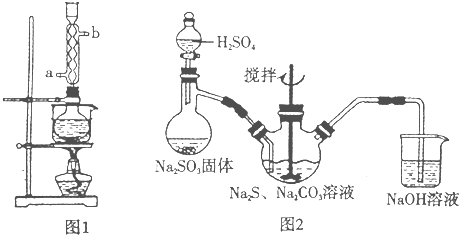
���������( Na2S2O3)�׳Ʊ��շۣ�����������ҵ����Ӱ����Ҳ������ֽ��Ư�����������ȡ�ʵ���ҿ�ͨ�����·�Ӧ��ȡ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2��
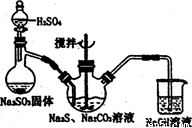
ͼl ͼ2
(1)��ͼl��ʾװ����ȡNa2S2O3������NaOH��Һ�������ǣ� ��
�罫��Һ©���е�H2SO4�ij�Ũ���ᣬ��������ƿ�ڳ�Na2S2O3�����⣬���� (�ѧʽ)�������ɡ�
Ϊ�ⶨ���ñ������Ʒ��Na2S2O3��5H2O���������������ñ�����Һ���еζ�����Ӧ����ʽΪ2 Na2S2O3+I2=2NaI+Na2S4O6��
(2)����KIO3��KI��HCI�����Ʊ�����Һ��д������ʱ��������Ӧ�����ӷ���ʽ�� ��
(3)ȷ��ȡһ��������Na2S2O3��5H2O��Ʒ����ƿ�У���ˮ�ܽ⣬���μ� ��ָʾ�����������Ƶı�����Һ�ζ����ζ�ʱ���õIJ�����������ƿ�⣬���� ��
(4)���춨ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�춨�����ʹ��Ʒ��Na2S2O3��5H2O�����������IJ������____(�ƫ�ߡ�ƫ�͡����䡱)��
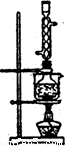
(5)��ʵ���Na2S�Ĵ���Ҫ��ϸߣ�����ͼ2��ʾ��װ�ÿɽ���ҵ����Na2S�ᴿ��
��֪Na2S���������ھƾ�������ʱ�ܽ��Ѹ���������ʲ����ھƾ����ᴿ��������Ϊ��
�ٽ��ѳ����õĹ�ҵNa2S����Բ����ƿ�У�������һ�������ľƾ�������ˮ��
�ڰ�ͼ2��ʾװ����������������������ͨ����ȴˮ��ˮԡ���ȣ�
�۴���ƿ�й��岻�ټ���ʱ��ֹͣ���ȣ�����ƿȡ�¡�
�� ��
�� ��
�����ù���ϴ�ӡ�����õ�Na2S��9H2O���塣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com