
| A�� | ���Թ�ȡ���Լ�ƿ�е�Na2CO3��Һ������ȡ�����࣬Ϊ�˲��˷ѣ��ְѹ������Լ������Լ�ƿ�� | |
| B�� | Ba��NO3��2����ˮ���ɽ�����Ba��NO3��2�ķ�Һ����ˮ���У�����ˮ������ˮ�� | |
| C�� | ����������ʹNaCl����Һ������ʱ��Ӧ����������NaCl��Һȫ���������� | |
| D�� | �ⶨ��ҺpH�IJ�������pH��ֽ���ڱ������ϣ��ò�����պȡ��Һ������pH��ֽ���в������Ӧ�ı���ɫ���Ƚ� |
���� A��һ������Լ����ܷŻ�ԭƿ����ֹ��Ⱦ��
B���������ж�������Ⱦ����ˮ��
C������ʱ�������ɣ��������ȼ��ȣ�
D��������պȡ��Һ������pH��ֽ���в����ⶨpH��
��� �⣺A��һ������Լ����ܷŻ�ԭƿ����ֹ��Ⱦ��Ӧ����ָ�����ձ��У���A����
B���������ж�������Ⱦ����ˮ�����ܽ�����Ba��NO3��2�ķ�Һ����ˮ���У�����ˮ������ˮ������B����
C������ʱ�������ɣ��������ȼ��ȣ�����ִ�������ʱֹͣ���ȣ���C����
D����pH��ֽ���ڱ������ϣ��ò�����պȡ��Һ������pH��ֽ���в������Ӧ�ı���ɫ���Ƚϣ��ⶨpH�IJ�����������D��ȷ��
��ѡD��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�������ʵ����ʡ�ʵ�������ʵ�鰲ȫ��ʵ�鼼��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��ʵ��������Է�������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶��Ͻ�ѧ�ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
�����ܼ�������ȷ����
A. 2d B. 3f C. 1p D. 6s
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
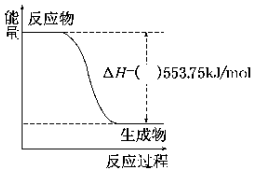 �������˻ᡰ�����������þ��й��������ɫ���������οչ��գ���ˮ�ĺ�г���ļ��齻������Ϊ�����������������ͬ�ƽ���ƽ�뷢չ����������������ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϣ��Իش��������⣺
�������˻ᡰ�����������þ��й��������ɫ���������οչ��գ���ˮ�ĺ�г���ļ��齻������Ϊ�����������������ͬ�ƽ���ƽ�뷢չ����������������ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϣ��Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Fe��OH��3�����м���HI��Һ��Fe��OH��3+3H+�TFe3++3H2O | |
| B�� | ��״���½�112ml����ͨ��6ml 1mol/L�ĵ⻯������Һ��3Cl2+2Fe2++4I-�T6Cl-+2Fe3++2I2 | |
| C�� | �����ʯ��ˮ�м�������NaHCO3��Һ��Ca2++OH-+HCO3-�TCaCO3��+H2O | |
| D�� | ��H2O2��H2SO4�Ļ����Һʴ��ͭ��Cu+H2O2+2H+�TCu2++2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4 Lˮ�������ķ�����ԼΪ6.02��1023�� | |
| B�� | 1 mol Cl2�к��е�ԭ����ΪNA | |
| C�� | ��״���£�a L�����͵����Ļ���ﺬ�еķ�����ԼΪ$\frac{a}{22.4}$��6.02��1023�� | |
| D�� | ���³�ѹ�£�11.2 L CO��������0.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.5 L | B�� | 0.1 L | C�� | 0.2 L | D�� | 1 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ԭ����ͭ | |
| B�� | ������������ȼ�� | |
| C�� | Ba��OH��2•8H2O��NH4Cl��Ӧ | |
| D�� | ̼��Ƹ��·ֽ�������ƺͶ�����̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 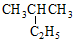 2-�һ����� 2-�һ����� | B�� | 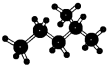 ������ ������ | ||
| C�� | 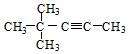 4��4-����-2-��Ȳ 4��4-����-2-��Ȳ | D�� | 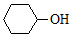 ������ ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com