
��ͼ�ǽ��ֳּ�������δ֪Ũ������ζ�20.00 mL 0.1 mol��L-1NaOH��Һ,�ⶨ����Ũ�ȵ�ʵ��(����ĵζ�����Ϊ0.098 mL��s-1)�������й�˵���в���ȷ����(����)
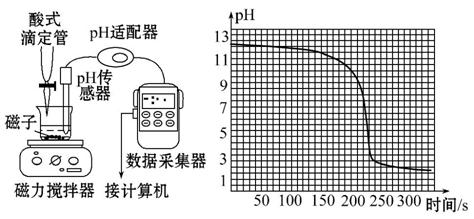
A.���к͵ζ��ķ�Ӧԭ����:H++OH-====H2O
B.H+��OH-��Ӧ����ˮʹ��Һ��pH�����仯,�ô������ܾ�ȷ�ⶨ����
C.�봫ͳ�к͵ζ��Ƚ�,���Ա������ָʾ�����յ�ȷ�жϵ�����
D.����ͼ�м�������Ƶ�����к͵ζ����߿�֪:250 sʱ�ﵽ�ζ��յ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϵ�ⷨ�����������Է�ˮ���õ�����Ni��ԭ����ͼ��ʾ������˵������ȷ����
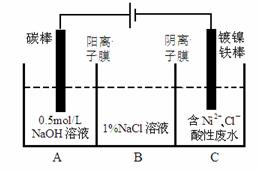
��֪�� ��Ni2+����������Һ�з���ˮ�� �������ԣ�Ni2+����Ũ�ȣ���H+��Ni2+����Ũ�ȣ�
A��̼���Ϸ����ĵ缫��Ӧ��4OH�� �� 4e�� == O2��+2H2O
B��Ϊ�����Ni�IJ��ʣ�����������Ҫ���Ʒ�ˮpH
C���������У�B��NaCl��Һ�����ʵ���Ũ�Ƚ����ϼ�С
D������ͼ��������Ĥȥ������A��B���Һϲ������ⷴӦ�ܷ���ʽ�����ı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��һ����������CO(g)+H2O(g) CO2(g)+H2(g)����ijһ�ݻ�Ϊ2 L���ܱ�������,����0.2 mol��CO��0.2 mol��H2O,�ڴ������ڵ�������,���¼���,�������·�Ӧ:CO(g)+H2O(g)====CO2(g)+H2(g)��
CO2(g)+H2(g)����ijһ�ݻ�Ϊ2 L���ܱ�������,����0.2 mol��CO��0.2 mol��H2O,�ڴ������ڵ�������,���¼���,�������·�Ӧ:CO(g)+H2O(g)====CO2(g)+H2(g)��
��H=akJ��mol-1��Ӧ��ƽ���,���c(CO)��c(CO2)=3��2,����˵����ȷ����
(����)
A.��Ӧ�ų�������Ϊ0.04akJ
B.ƽ��ʱH2O��ת����Ϊ40%
C.�������������ѹ��Ϊ1 L,�����ڸ÷�Ӧƽ�������ƶ�
D.�жϸ÷�Ӧ�ﵽƽ���������CO��H2O��CO2��H2��Ũ�ȶ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��pH=1������ƽ���ֳ�����,һ�ݼ�������ˮ,��һ�ݼ�������������ʵ���Ũ����ͬ������NaOH��Һ,pH��������1,������ˮ��NaOH��Һ�������Ϊ
��(����)
A.9 B.10 C.11 D.12
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.ʵ������һƿʧȥ��ǩ��ij��ɫ����,��֪��ɷֿ�����̼�������������λ����,����ɷֵ�һ����ij��ѧС��ͨ�����²�����ȷ����ɷ�:
(1)�����ӵ�ȷ��:
ʵ�鷽��������:�� ��
����:�˰�ɫ���������Ρ�
(2)�����ӵ�ȷ��:
��ȡ������ɫ�������Թ���,Ȼ�����Թ��м���ϡ����,��ɫ����ȫ���ܽ�,������ɫ����,��������ʹ��ˮ��ɫ��
��Ҫ��һ��ȷ����ɷ��貹������ʵ��:ȡ������ɫ���������Һ,ȡ������Һ���Թ���,����BaCl2��Һ,���ְ�ɫ������
��.ȷ����ɷֺ�,����ijЩԭ��,�˰�ɫ���岿�ֱ���������,�û�ѧС��������֪Ũ�ȵ�����KMnO4��Һ��ȷ�����ʹ�����X�ĺ���,���岽������:
�����ȡ��Ʒ1.000 g��
�������Ʒ�ܽ��,��ȫת�Ƶ�250 mL����ƿ��,����,���ҡ�ȡ�
�������ȡ25.00 mL��Ʒ��Һ��250 mL��ƿ��,��0.01 mol��L-1KMnO4����Һ�ζ����յ㡣
�����������������ظ�2�Ρ�
(1)д���������������Ӧ�����ӷ���ʽ�� ��
(2)������0.01 mol��L-1KMnO4��Һʱ�����Ӷ���,�����ղ�ñ��ʹ�����X�ĺ�����������(�ƫ��ƫС������Ӱ�족)��
(3)�ζ�������±���ʾ:
| �ζ� ���� | ������Һ�� ���/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.20 | 20.20 |
��ñ��ʹ�����X����������Ϊ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ԭ�����Ӱڵ������������ȷ���ӡ���Ԫ��(Ԫ�ط���ΪSr)��ԭ�ӽṹʾ��ͼ��ͼ��ʾ������˵����ȷ����( )
A.�����ȵĻ�ѧʽΪSr2N3 B.�ȿ���ˮ��Ӧ����O2
C.��������Ϊ���� D.��һ�����ܣ�Sr<Ca
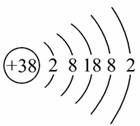
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�йص������������������ȷ����(����)
A���������������ʵ���һ�־ۼ�״̬
B�����������Ǻܺõĵ���
C��ˮ�������γɵ�������״̬
D�������������;ʮ�ֹ㷺
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���A1��A2�ֱ��Ũ������һ���¶��¹���ֻ������B��B�������ܶ���ͬ��ͬѹ��H2�ܶȵ�59�����ڴ��������£�1mol B���Ժ�4mol H2�����ӳɷ�Ӧ��B��һԪ��������������(ͬ������)���й�����֮���ת����ϵ������F�ķ���ʽΪC9H10O3�����£�
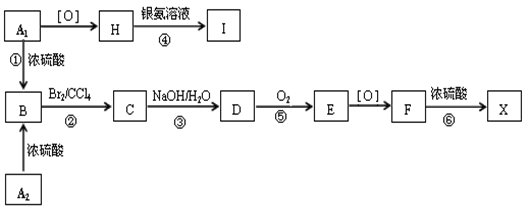
��ش��������⣺
��1�� ��Ӧ�� �� ��������ȡ����Ӧ���ǣ�_______________��
��2�� д��A2��F�����ʵĽṹ��ʽ��A2 ______________��F_____________________��
��3�� д���ۡ���������Ӧ�Ļ�ѧ����ʽ��
_______________________________________________________________��
_______________________________________________________________��
��4�� ������E�ж���ͬ���칹�壬�������������Ҿ���������λ������ͬ���칹��Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com