
������Ũ����ͬ���������������ҺA��B��AΪ���ᣬBΪ���ᣬ�ֱ��п��Ӧ����������һ����Һ�д���п���ҷų�������������ͬ��������˵����ȷ���ǣ� ��
�ٷ�Ӧ�����ʱ��B>A �ڿ�ʼ��Ӧʱ������ΪA>B �ۼ���п�����ʵ���A>B
�ܷ�Ӧ�����е�ƽ������ΪB>A ����������пʣ�� ��������пʣ��
A �ۢܢ� B �ۢܢ� C �ڢۢ� D �ڢۢݢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||


 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 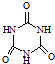 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ��| ������ | �ܶ�/g?cm-3 | �е�/�� | �ܽ��/100gˮ |
| ������ | 0.810 | 118.0 | 9 |
| ������ | 1.049 | 118.1 | �� |
| ���������� | 0.882 | 126.1 | 0.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| �� |
| �� |
| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2012�����������¿���ѧ���� ���ͣ�043
| |||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�ߴ�����ѧ2008�����������⻯ѧ�Ծ� ���ͣ�022
��ͼ��Ԫ�����ڱ��е�һ���֣�����������ĸ�ֱ����һ�ֻ�ѧԪ�أ���ش��������⣺
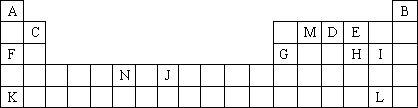
(1)��������Ԫ�ص�ԭ���У��������Ӳ���ֻ���������ӵ���________(�Ԫ�ط��š�)��
(2)����������ȷ����________(����ĸ���)��
a��K������������Ӧ��ˮ������һ��ǿ��
b����������ȶ���ǿ��H���⻯������ȶ���
c��F��H�γɻ�����ˮ��Һ��pH����F��I�γɻ�����ˮ��Һ��pH��˵����ͬŨ���⻯��ˮ��Һ������H����I
d��MA2E������Mԭ�Ӳ�ȡSP2�ӻ���ʽ
e��N�ĵ����Ų�ʽ��ls22s22p63s23p63d44s2
(3)����VSEPR����Ԥ��DA4+���ӵĿռ乹��________��ME2�Ŀռ乹��________��
(4)Ԫ��F��Ԫ��I�γɵĻ�������________����(�������)����ͼΪ�þ���Ľṹʾ��ͼ������ͼ����ʵ����(��)�Ϳ�����(��)�ֱ��ʾԪ��F��Ԫ��I����λ�ã�
(5)��֪Ti3+���γ���λ��Ϊ6���������к��ѵ�������ɫ�ľ��壬һ��Ϊ��ɫ����һΪ��ɫ�����ʵ��֤�������־������ɽ�ΪTiCl3��6H2O��Ϊ�ⶨ�����־���Ļ�ѧʽ�����������ʵ�飺
a���ֱ�ȡ����������������ᄃ�����Ʒ��ɴ�����Һ��
b���ֱ���������Һ�е���AgNO3��Һ����������ɫ������
c��������ȫ��ֱ���˵����ݳ�������ϴ�Ӹ�������������ԭ��ɫ�����ˮ��Һ��AgNO3��Һ��Ӧ�õ��İ�ɫ��������Ϊ��ɫ�����ˮ��Һ��Ӧ�õ�����������2/3��
����ɫ����Ļ�ѧʽΪ________����������е���λ��Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com