

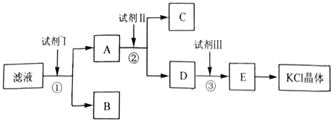
·ÖĪö £Ø1£©ŅĄ¾ŻÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄ²½ÖčŃ”ŌńŠčŅŖµÄŅĒĘ÷£»
£Ø2£©ŅĄ¾ŻÅäÖĘČÜŅŗĢå»żŃ”ŌńŗĻŹŹµÄČŻĮæĘ棬ŅĄ¾Żm=CVM¼ĘĖćŠčŅŖČÜÖŹµÄÖŹĮ棻
£Ø3£©øł¾Ż×óĀėÓŅĪļ³ĘĮæŅ©Ę·Ź±£¬Ņ©Ę·µÄŹµ¼ŹÖŹĮæ=ķĄĀėµÄÖŹĮæ-ÓĪĀėµÄÖŹĮ棻
£Ø4£©ČŻĮæĘæŹ¹ÓĆ¹ż³ĢÖŠŠčŅŖÉĻĻĀµßµ¹£¬Ņ”¶Æ£»
£Ø5£©·ÖĪö²»µ±²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄŅ»°ć²½Öč£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ²Ł×÷£¬ÓƵ½µÄŅĒĘ÷ÓŠ£ŗĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢²£Į§°ō”¢500mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£»
±ŲŠėÓƵ½µÄ²£Į§ŅĒĘ÷ĪŖ£ŗÉÕ±”¢²£Į§°ō”¢500mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£»
¹Ź“š°øĪŖ£ŗÉÕ±£»²£Į§°ō£»500mLČŻĮæĘ棻½ŗĶ·µĪ¹Ü£»
£Ø2£©ÓĆNaOH¹ĢĢåÅäÖĘ2.0mol/LµÄNaOHČÜŅŗ480mL£¬Ó¦Ń”Ōń500mLČŻĮæĘ棬ŠčŅŖĒāŃõ»ÆÄʵÄÖŹĮæm=2.0mol/L”Į0.5L”Į40g/mol=40.0g£¬
¹Ź“š°øĪŖ£ŗ40.0£»
£Ø3£©Ķ¼ÖŠĪļÖŹŗĶķĄĀėµÄĪ»ÖĆ·Å·“ĮĖ£¬ÖŹĮæ¹ŲĻµÓ¦ĪŖ£ŗķĄĀėµÄÖŹĮæ=ÉÕ±µÄÖŹĮæ+ÓĪĀėĻŌŹ¾µÄÖŹĮ森¼“30 g=ÉÕ±µÄÖŹĮæ+2.6 g£¬µĆÉÕ±µÄÖŹĮæĪŖ£ŗ30g-2.6g=27.4g£»
¹Ź“š°øĪŖ£ŗ27.4£»
£Ø4£©ČŻĮæĘæŹ¹ÓĆ¹ż³ĢÖŠŠčŅŖÉĻĻĀµßµ¹£¬Ņ”¶Æ£¬ĖłŅŌŌŚŹ¹ÓĆĒ°±ŲŠė¼ģ²éŹĒ·ńĀ©Ė®£»
¹Ź“š°øĪŖ£ŗ¼ģ²éČŻĮæĘæŹĒ·ńĀ©Ė®£»
£Ø5£©A£®Ć»ÓŠĻ“µÓÉÕ±ŗĶ²£Į§°ō£¬µ¼ÖĀČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹA“ķĪó£»
B£®Ī“ĄäČ“µ½ŹŅĪĀ¾Ķ½«ČÜŅŗ×ŖŅʵ½ČŻĮæĘæ²¢¶ØČŻ£¬ĄäČ“ŗó£¬ČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹBÕżČ·£»
C£®ČŻĮæĘæ²»øÉŌļ£¬ŗ¬ÓŠÉŁĮæÕōĮóĖ®£¬¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗµÄĢå»ż¶¼²»»į²śÉśÓ°Ļģ£¬ČÜŅŗÅØ¶Č²»±ä£¬¹ŹC“ķĪó£»
D£®¶ØČŻŗóČūÉĻĘæČū·“ø“Ņ”ŌČ£¬¾²ÖĆŗó£¬ŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ŌŁ¼ÓĖ®ÖĮæĢ¶Č£¬µ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹD“ķĪó£»
¹ŹŃ”£ŗB£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ£¬ŹģĻ¤ÅäÖĘŌĄķŗĶ¹ż³ĢŹĒ½āĢā¹Ų¼ü£¬×¢ŅāĪó²ī·ÖĪö·½·ØŗĶ¼¼ĒÉ£¬ĢāÄæÄŃ¶Č²»“ó£®


 Ó¦ÓĆĢāµć²¦ĻµĮŠ“š°ø
Ó¦ÓĆĢāµć²¦ĻµĮŠ“š°ø דŌŖ¼°µŚĻµĮŠ“š°ø
דŌŖ¼°µŚĻµĮŠ“š°ø Ķ¬²½°ĀŹżĻµĮŠ“š°ø
Ķ¬²½°ĀŹżĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŃōĄė×Ó | Na+”¢K+”¢Cu2+ |
| ŅõĄė×Ó | SO42-”¢OH- |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na2CO3 | B£® | NaHCO3 | ||
| C£® | Na2CO3ŗĶNaHCO3 | D£® | NaOH”¢Na2CO3ŗĶNaHCO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH3 | B£® | CH4 | C£® | NaHCO3 | D£® | HNO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé²Ł×÷¼°ĻÖĻó | ŹµŃé½įĀŪ |
| A | ĻņijÄĘŃĪÖŠ¼ÓČėĻ”ŃĪĖį£¬²śÉśÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢå | ĖµĆ÷øĆŃĪŅ»¶ØŹĒĢ¼ĖįŃĪ |
| B | ĻņijĪŽÉ«ČÜŅŗÖŠµĪ¼ÓBaCl2ČÜŅŗ£¬ŌŁµĪ¼Ó¹żĮæµÄĻ”HNO3£¬²śÉś°×É«³Įµķ | øĆĪŽÉ«ČÜŅŗÖŠŅ»¶ØÓŠSO42- |
| C | ĻņijĪŽÉ«ČÜŅŗÖŠµĪ¼ÓNaOHČÜŅŗ£¬¼ÓČČŗ󣬲śÉśŹĒŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢå | ĖµĆ÷ČÜŅŗŅŗÖŠŗ¬ÓŠNH4+ |
| D | ijĪŽÉ«ĘųĢåĶعż×ĘČȵÄCuO£¬CuO±äĪŖŗģÉ« | øĆĘųĢåŅ»¶ØĪŖĒāĘų |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 0.3 mol•L-1 K2SO4ČÜŅŗÖŠŗ¬ÓŠ0.6NAøöK+ŗĶ0.3 molµÄSO42- | |
| B£® | ŌŚ±ź×¼×“æöĻĀH2OµÄĦ¶ūĢå»żŌ¼ŹĒ22.4 L•mol-1 | |
| C£® | ½«58.5gµÄNaCl¹ĢĢåČܽāŌŚ1LµÄĖ®ÖŠ£¬ĖłµĆµÄČÜŅŗĪļÖŹµÄĮæÅضČĪŖ1mol/L | |
| D£® | 1 molČĪŗĪĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»ż¶¼Ō¼ĪŖ22.4 L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
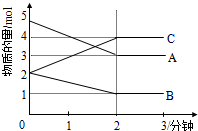 £Ø1£©Ä³æÉÄę·“Ó¦£ØABC¾łĪŖĘųĢ壩“Ó0-2·ÖÖÓ½ųŠŠ¹ż³ĢÖŠ£¬ŌŚ²»Ķ¬·“Ó¦Ź±¼äø÷ĪļÖŹµÄĮæµÄ±ä»ÆĒéæöČēĶ¼ĖłŹ¾£®
£Ø1£©Ä³æÉÄę·“Ó¦£ØABC¾łĪŖĘųĢ壩“Ó0-2·ÖÖÓ½ųŠŠ¹ż³ĢÖŠ£¬ŌŚ²»Ķ¬·“Ó¦Ź±¼äø÷ĪļÖŹµÄĮæµÄ±ä»ÆĒéæöČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com