
| A£® | ³£ĪĀĻĀ0.4 mol/L HBČÜŅŗŗĶ 0.2 mol/L NaOHČÜŅŗµČĢå»ż»ģŗĻŗóČÜŅŗµÄpH=3£¬Ōņ»ģŗĻČÜŅŗÖŠĄė×ÓÅØ¶ČµÄ“óŠ”Ė³ŠņĪŖ£ŗc£ØB-£©£¾c£ØH+£©£¾c£ØNa+£©£¾c£ØOH-£© | |
| B£® | µČÅØ¶ČµÄĻĀĮŠĻ”ČÜŅŗ£ŗ¢ŁĮņĖįĒāÄĘ ¢ŚŅŅĖįÄĘ ¢Ū“×Ėį ¢ÜĢ¼ĖįĒāÄĘ ¢ŻĻõĖįÄĘ ¢Ž±½·ÓÄĘ£¬ĖüĆĒµÄpHÓÉŠ”µ½“óÅÅĮŠĪŖ£ŗ¢Ū¢Ż¢Ł¢Ü¢Ś¢Ž | |
| C£® | ³£ĪĀĻĀ0.1 mol/LµÄĻĀĮŠČÜŅŗ ¢ŁNH4Al£ØSO4£©2 ¢ŚNH4Cl ¢ŪNH3•H2O ¢ÜCH3COONH4ÖŠc £ØNH4+£©Óɓ󵽊”µÄĖ³ŠņŹĒ£ŗ¢Ś£¾¢Ł£¾¢Ü£¾¢Ū | |
| D£® | ŌŚ25”ꏱ£¬½«a mol•L-1µÄ°±Ė®Óė0.01 mol•L-1µÄŃĪĖįµČĢå»ż»ģŗĻ·“Ó¦Ź±ČÜŅŗÖŠc£ØNH4+£©=c£ØCl-£©£®ÓĆŗ¬aµÄ“śŹżŹ½±ķŹ¾NH3•H2OµÄµēĄė³£ŹżKb=$\frac{1{0}^{-9}}{a-0.01}$ |
·ÖĪö A£®0.4mol/LHBČÜŅŗŗĶ0.2mol/LNaOHČÜŅŗµČĢå»ż»ģŗĻŗó·¢Éś·“Ó¦£¬ŌņŹµÖŹÉĻŹĒ0.1mol/LHBČÜŅŗŗĶ0.1mol/L NaBČÜŅŗ£¬øĆČÜŅŗĻŌĖįŠŌ£¬Č»ŗóĄūÓƵēŗÉŹŲŗć¼°ĪļĮĻŹŲŗćµČĄ“·ÖĪöĄė×ÓÅØ¶ČµÄ¹ŲĻµ£»
B£®ĻČ°“ÕÕĪļÖŹµÄĄą±š·ÖĪŖ¼ī”¢ŃĪ”¢Ėį½ųŠŠ·ÖĄą£¬ŌŚ“Ė»ł“”ÉĻÖ÷ŅŖæ¼ĀĒŃĪµÄĖ®½ā£¬ÅŠ¶ĻŃĪČÜŅŗµÄĖį¼īŠŌŌŁ½ųŠŠ±Č½Ļ£»
C£®¢ŁĀĮĄė×ÓĖ®½āŅÖÖĘļ§øłĄė×ÓµÄĖ®½ā£»¢Śļ§øłĄė×ÓĖ®½ā£»¢ŪČõ¼īµēĄė£¬ĒŅµēĄėµÄ³Ģ¶ČŗÜČõ£»¢Ü“×ĖįøłĄė×ÓĖ®½ā“Ł½ųļ§øłĄė×ÓĖ®½ā£»
D£®ŌŚ25”ęĻĀ£¬Ę½ŗāŹ±ČÜŅŗÖŠc£ØNH4+£©=c£ØCl-£©=0.005mol/L£¬øł¾ŻĪļĮĻŹŲŗćµĆc£ØNH3£®H2O£©=£Ø0.5a-0.005£©mol/L£¬øł¾ŻµēŗÉŹŲŗćµĆc£ØH+£©=c£ØOH-£©=10-7mol/L£¬ČÜŅŗ³ŹÖŠŠŌ£¬½įŗĻNH3•H2OµÄµēĄė³£Źż±ķ“ļŹ½¼ĘĖć£®
½ā“š ½ā£ŗA£®»ģŗĻŗóĪŖ0.1mol/LHBČÜŅŗŗĶ0.1mol/L NaBČÜŅŗ£¬ČÜŅŗµÄPH=3£¬ČÜŅŗ³ŹĖįŠŌ£¬ĖłŅŌĖįµÄµēĄė“óÓŚŃĪµÄĖ®½ā£¬Ōņc£ØB-£©£¾c£ØHB£©£¬ÄĘĄė×ÓŹĒ0.1mol/L£¬Ōņc£ØB-£©£¾c£ØNa+£©£¾c£ØHB£©£¬ČÜŅŗ³ŹĖįŠŌĖµĆ÷ČÜŅŗÖŠc£ØH+£©£¾c£ØOH-£©£¬µ«ČÜŅŗÖŠµÄĒāĄė×ÓŗĶĒāŃõøłĄė×ÓÅØ¶Č¶¼½ĻŠ”£¬Š”ÓŚĖįµÄÅØ¶Č£¬ĖłŅŌĄė×ÓÅØ¶Č“óŠ”Ė³ŠņŹĒc£ØB-£©£¾c£ØNa+£©£¾c£ØHB£©£¾c£ØH+£©£¾c£ØOH-£©£¬¹ŹA“ķĪó£»
B£®µČÅØ¶ČŹ±£¬¢ŁĮņĖįĒāÄĘĻąµ±ÓŚŅ»ŌŖĒæĖį£¬¢Ū“×ĖįĪŖŅ»ŌŖČõĖį£¬¢Ž±½·ÓÄĘ”¢¢ÜĢ¼ĖįĒāÄĘ”¢¢ŚŅŅĖįÄĘĖ®½ā¶¼³Ź¼īŠŌ£¬ĒŅĖ®½ā³Ģ¶ČŅĄ“Ī¼õŠ”£¬ĖłŅŌČÜŅŗpH¢Ž£¾¢Ü£¾¢Ś£¬¢ŻĻõĖįÄĘČÜŅŗ³ŹÖŠŠŌ£¬pH=7£¬ĖłŅŌČÜŅŗpHÓÉŠ”µ½“óµÄĖ³ŠņĪŖ¢Ł¢Ū¢Ż¢Ś¢Ü¢Ž£¬¹ŹB“ķĪó£»
C£®Ķ¬ÅØ¶ČµÄĻĀĮŠČÜŅŗ£ŗ¢ŁNH4Al£ØSO4£©2¢ŚNH4Cl¢ŪNH3•H2O£¬¢ÜCH3COONH4£¬Ņņ¢ŁÖŠĀĮĄė×ÓĖ®½āŅÖÖĘļ§øłĄė×ÓµÄĖ®½ā£»¢ŚÖŠļ§øłĄė×ÓĖ®½ā£»¢ŪČõ¼īµēĄė£¬ĒŅµēĄėµÄ³Ģ¶ČŗÜČõ£»¢Ü“×ĖįøłĄė×ÓĖ®½ā“Ł½ųļ§øłĄė×ÓĖ®½ā£¬Ōņc£ØNH4+£©Óɓ󵽊”µÄĖ³ŠņŹĒ£ŗ¢Ł£¾¢Ś£¾¢Ü£¾¢Ū£¬¹ŹC“ķĪó£»
D£®ŌŚ25”ęĻĀ£¬Ę½ŗāŹ±ČÜŅŗÖŠc£ØNH4+£©=c£ØCl-£©=0.005mol/L£¬øł¾ŻĪļĮĻŹŲŗćµĆc£ØNH3£®H2O£©=£Ø0.5a-0.005£©mol/L£¬øł¾ŻµēŗÉŹŲŗćµĆc£ØH+£©=c£ØOH-£©=10-7mol/L£¬ČÜŅŗ³ŹÖŠŠŌ£¬NH3•H2OµÄµēĄė³£ŹżKb=$\frac{c£ØO{H}^{-}£©£®c£ØN{{H}_{4}}^{+}£©}{c£ØN{H}_{3}•{H}_{2}O£©}$=$\frac{1{0}^{7}”Į5”Į1{0}^{-3}}{0.5a-5”Į1{0}^{-3}}$=$\frac{1{0}^{-9}}{a-0.01}$£¬¹ŹDÕżČ·£»
¹ŹŃ”D£®
µćĘĄ ±¾Ģāæ¼²éŃĪĄąĖ®½ā”¢Ąė×ÓÅØ¶Č“óŠ”±Č½Ļ”¢µēĄėĘ½ŗā³£ŹżµÄ¼ĘĖćµČÖŖŹ¶£¬ĢāÄæÄѶČÖŠµČ£¬×¢ŅāŹģĮ·ĄūÓƵēŗÉŹŲŗć”¢ĪļĮĻŹŲŗćŗĶÖŹ×ÓŹŲŗ楓½āĢā£¬²ąÖŲÓŚæ¼²éѧɜµÄ·ÖĪöÄÜĮ¦ŗĶ¶Ō»ł“”ÖŖŹ¶µÄ×ŪŗĻÓ¦ÓĆÄÜĮ¦£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
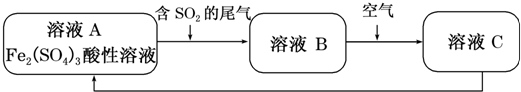
| A£® | ĻņCČÜŅŗÖŠµĪ¼ÓKSCNČÜŅŗ£¬ČÜŅŗ±äĪŖŃŖŗģÉ« | |
| B£® | ČÜŅŗB×Ŗ»ÆĪŖČÜŅŗC·¢ÉśµÄ±ä»ÆµÄĄė×Ó·½³ĢŹ½ĪŖ4H++2Fe2++O2ØT2Fe3++2H2O | |
| C£® | ČÜŅŗĖįŠŌA£¾B£¾C | |
| D£® | ¼ÓŃõ»ÆŃĒĢśæÉŅŌŹ¹ČÜŅŗC×Ŗ»ÆĪŖČÜŅŗA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
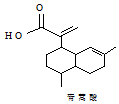
| A£® | ĒąŻļĖįµÄ·Ö×ÓŹ½ĪŖC15H20O2 | |
| B£® | 1molĒąŻļĖį×ī¶ąæÉÓė3molH2¼Ó³É | |
| C£® | ĒąŻļĖįÓė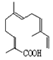 »„ĪŖĶ¬·ÖŅģ¹¹Ģå »„ĪŖĶ¬·ÖŅģ¹¹Ģå | |
| D£® | ±½»·ÉĻĮ¬ÓŠ-CHOŗĶ-OH”¢·Ö×ÓÖŠÓŠ6ÖÖ²»Ķ¬»Æѧ»·¾³ĒāµÄĒąŻļĖįµÄĶ¬·ÖŅģ¹¹ĢåÖ»ÓŠ2ÖÖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 3.2gCH3OHŗ¬ÓŠµÄ»Æѧ¼üŹżĪŖ0.4NA | |
| B£® | 0.1molFeCl3Ė®½āÖʵƵÄFe£ØOH£©3½ŗĢåÖŠ½ŗĮ£ŹżŹĒ0.1NA | |
| C£® | ±ź×¼×“æöĻĀ£¬2.24LCl2ČÜÓŚ×ćĮæĖ®£¬×ŖŅʵĵē×ÓŹżĪŖ0.1NA | |
| D£® | 0.2gD216OÖŠŗ¬ÓŠµÄÖŹ×ÓŹż”¢ÖŠ×ÓŹżŗĶµē×ÓŹż¾łĪŖ0.1NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | a=$\frac{PM}{2000¦Ń}$ | B£® | P=$\frac{200¦Ńa}{M}$ | C£® | P$\frac{1000¦Ń”Įa%}{M}$”Į2 | D£® | V”Į¦Ń”Įa%=$\frac{PMV}{1000}$ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŃōĄė×Ó | K+”¢NH4+”¢Fe3+”¢Ba2+ |
| ŅõĄė×Ó | Cl-”¢Br-”¢CO32-”¢HCO3-”¢SO32-”¢SO42- |
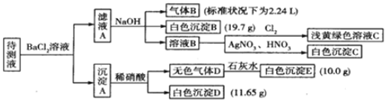
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com