
ŹµŃéŹŅÓĆČ¼Éշزā¶ØijÖÖ°±»łĖį(CxHyOzNp)µÄ·Ö×Ó×é³É”£Č”W gøĆÖÖ°±»łĖį·ÅŌŚ“æŃõÖŠ³ä·ÖČ¼ÉÕ£¬Éś³É¶žŃõ»ÆĢ¼”¢Ė®ŗĶµŖĘų”£ĻÖ°“Ķ¼1-3×°ÖĆ½ųŠŠŹµŃé£ŗ
Ķź³ÉĻĀĮŠĪŹĢā£ŗ
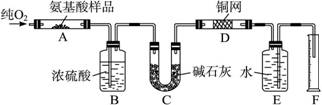
Ķ¼1-3
(1)ŹµŃéæŖŹ¼Ź±£¬Ź×ĻČŅŖĶØČėŅ»¶ĪŹ±¼äµÄŃõĘų£¬ĘäĄķÓÉŹĒ______________________________”£
(2)ŅŌÉĻ×°ÖĆÖŠŠčŅŖ¼ÓČȵÄŅĒĘ÷ÓŠ___________________________£¬²Ł×÷Ź±Ó¦ĻȵćČ¼_______“¦µÄ¾Ę¾«µĘ”£(ĢīŠ“×ÖÄø)
(3)A×°ÖĆÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_____________________________________________”£
(4)×°ÖĆDµÄ×÷ÓĆŹĒ_____________________________________________________________”£
(5)¶ĮČ”µŖĘųĢå»żŹ±£¬Ó¦×¢Ņā______________________________________________________”£
(6)ŹµŃéÖŠ²āµĆµŖĘųµÄĢå»żĪŖV mL(±ź×¼×“æö)£¬ĪŖČ·¶Ø“Ė°±»łĖįµÄ·Ö×ÓŹ½£¬»¹ŠčŅŖµÄÓŠ¹ŲŹż¾ŻÓŠ______________________”£
A.Éś³É¶žŃõ»ÆĢ¼µÄÖŹĮæ B.Éś³ÉĖ®µÄÖŹĮæ
C.ĶØČėŃõĘųµÄĢå»ż D.°±»łĖįµÄĻą¶Ō·Ö×ÓÖŹĮæ
(1)Åž»×°ÖĆÄŚµÄN2
£Ø2£©AD D
£Ø3£©CxHyOxNp+(x+![]() -
-![]() )O2
)O2![]() xCO2+
xCO2+![]() H2O+
H2O+![]() N2
N2
£Ø4£©ĪüŹÕĪ“·“Ó¦µÄŃõĘų£¬±£Ö¤×īÖÕŹÕ¼ÆµÄŹĒN2
£Ø5£©ĮæĶ²ĄļµÄŅŗĆęÓė¹ćæŚĘæÖŠµÄŅŗĆęĻą³ÖĘ½£¬ŹÓĻßÓ¦ÓėĮæĶ²æĢ¶ČŗĶ°¼ŅŗĆę×īµĶµćĻąĒŠ
£Ø6£©ABD
×öŹµŃéĢāŹ±Ź×ĻČŅŖĒ峞ŹµŃéµÄÄæµÄ”¢ŌĄķ”¢ŅĒĘ÷£¬Č»ŗó½įŗĻŅŃ¾ÕĘĪÕµÄŹµŃé²Ł×÷ÖŖŹ¶ŗĶĻą¹ŲĪļÖŹµÄŠŌÖŹµČÖŖŹ¶£¬°“ÕÕĢāÄæŅŖĒó½ā“š”£


 ÓÅÖŹæĪĢĆæģĄÖ³É³¤ĻµĮŠ“š°ø
ÓÅÖŹæĪĢĆæģĄÖ³É³¤ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)ŹµŃéæŖŹ¼Ź±£¬Ź×ĻČŅŖĶØČėŅ»¶ĪŹ±¼äµÄŃõĘų£¬ĘäĄķÓÉŹĒ______________________”£
(2)ŅŌÉĻ×°ÖĆÖŠŠčŅŖ¼ÓČȵÄŅĒĘ÷ÓŠ______________________(ÓĆ×ÖÄøĢīæÕ£¬ĻĀĶ¬)”£²Ł×÷Ź±Ó¦ĻȵćČ¼__________“¦µÄ¾Ę¾«µĘ”£
(3)A×°ÖĆÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____________________________”£
(4)×°ÖĆDµÄ×÷ÓĆŹĒ_____________________________”£
(5)¶ĮČ”N2µÄĢå»żŹ±£¬Ó¦×¢Ņā¢Ł______________________£»¢Ś______________________”£
(6)ŹµŃéÖŠ²āµĆN2µÄĢå»żĪŖV mL(±ź×¼×“æö)”£ĪŖČ·¶Ø“Ė°±»łĖįµÄ»ÆѧŹ½£¬»¹ŠčŅŖµÄÓŠ¹ŲŹż¾ŻŹĒ_____________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ½Ī÷ÉĻČÄ֊ѧøßŅ»Ē±ÄÜ°ąĻĀĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
(14·Ö)ŹµŃéŹŅÓĆČ¼Éշزā¶Øij¹ĢĢåÓŠ»śĪļAµÄ·Ö×Ó×é³É£¬²ā¶Ø×°ÖĆČēĶ¼(Ģś¼ÜĢØ”¢Ģś¼Š”¢¾Ę¾«µĘµČĪ“»³ö)£ŗ
Č”17.1 g A·ÅČė×°ÖĆÖŠ£¬ĶØČė¹żĮæO2Č¼ÉÕ£¬Éś³ÉCO2ŗĶH2O£¬Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā£ŗ
(1)ĶØČė¹żĮæO2µÄÄæµÄŹĒ______________________________________________”£
(2)C×°ÖƵÄ×÷ÓĆŹĒ__________________________________________£»
D×°ÖƵÄ×÷ÓĆŹĒ_____________________________________________”£
(3)ĶعżøĆŹµŃ飬ÄÜ·ńČ·¶ØAÖŠŹĒ·ńŗ¬ÓŠŃõŌ×Ó£æ________”£
(4)ČōAµÄĦ¶ūÖŹĮæĪŖ342 g/mol£¬C×°ÖĆŌöÖŲ9.99 g£¬D×°ÖĆŌöÖŲ26.4 g£¬ŌņA·Ö×ÓŹ½ĪŖ____________”£
(5)Š“³öAČ¼ÉյĻÆѧ·½³ĢŹ½_____________________________________”£
(6)AæÉ·¢ÉśĖ®½ā·“Ó¦£¬1 mol AæÉĖ®½āÉś³É2 molĶ¬·ÖŅģ¹¹Ģ壬ŌņAŌŚ“߻ƼĮ×÷ÓĆĻĀĖ®½āµÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ½Ī÷ÉĻČÄ֊ѧøßŅ»Ē±ÄÜ°ąĻĀĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
(14·Ö)ŹµŃéŹŅÓĆČ¼Éշزā¶Øij¹ĢĢåÓŠ»śĪļAµÄ·Ö×Ó×é³É£¬²ā¶Ø×°ÖĆČēĶ¼(Ģś¼ÜĢØ”¢Ģś¼Š”¢¾Ę¾«µĘµČĪ“»³ö)£ŗ

Č”17.1 g A·ÅČė×°ÖĆÖŠ£¬ĶØČė¹żĮæO2Č¼ÉÕ£¬Éś³ÉCO2ŗĶH2O£¬Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā£ŗ
(1)ĶØČė¹żĮæO2µÄÄæµÄŹĒ______________________________________________”£
(2)C×°ÖƵÄ×÷ÓĆŹĒ__________________________________________£»
D×°ÖƵÄ×÷ÓĆŹĒ_____________________________________________”£
(3)ĶعżøĆŹµŃ飬ÄÜ·ńČ·¶ØAÖŠŹĒ·ńŗ¬ÓŠŃõŌ×Ó£æ________”£
(4)ČōAµÄĦ¶ūÖŹĮæĪŖ342 g/mol£¬C×°ÖĆŌöÖŲ9.99 g£¬D×°ÖĆŌöÖŲ26.4 g£¬ŌņA·Ö×ÓŹ½ĪŖ____________”£
(5)Š“³öAČ¼ÉյĻÆѧ·½³ĢŹ½_____________________________________”£
(6)AæÉ·¢ÉśĖ®½ā·“Ó¦£¬1 mol AæÉĖ®½āÉś³É2 molĶ¬·ÖŅģ¹¹Ģ壬ŌņAŌŚ“߻ƼĮ×÷ÓĆĻĀĖ®½āµÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĶ¬²½Ģā ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹŅÓĆČ¼Éշزā¶Øij°±»łĖįCxHyOzNpµÄ·Ö×Ó×é³É”£Č”mgøĆÖÖ°±»łĖį·ÅŌŚ“æŃõÖŠ³ä·ÖČ¼ÉÕ£¬Éś³ÉCO2”¢H2OŗĶN2£¬ŹµŃé×°ÖĆČēĻĀĶ¼£ŗ

Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©ŹµŃéæŖŹ¼Ź±£¬Ź×ĻČŅŖĶØČėŅ»¶ĪŹ±¼äµÄŃõĘų£¬ĘäĄķÓÉŹĒ£ŗ
£¬
£Ø2£©ŅŌÉĻ×°ÖĆÖŠŠčŅŖ¼ÓČȵÄŅĒĘ÷ÓŠ £ØĢī×ÖÄø£¬ĻĀĶ¬£©²Ł×÷Ź±Ó¦ĻȵćČ¼
“¦µÄ¾Ę¾«µĘ”£
£Ø3£©A×°ÖĆÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ
£Ø4£©×°ÖĆDµÄ×÷ÓĆŹĒ£ŗ
£Ø5£©¶ĮČ”N2Ģå»żŹ±£¬Ó¦×¢Ņā£ŗ¢Ł £»
¢Ś ”£
£Ø6£©ŹµŃéÖŠ²āµĆN2µÄĢå»żVmL£ØŅŃÕŪĖćĪŖ±ź×¼×“æö£©£¬ĪŖČ·¶Ø“Ė°±»łĖįµÄ·Ö×ÓŹ½£¬»¹ŠčŅŖµÄÓŠ¹ŲŹż¾ŻÓŠ £ØÓĆ×ÖÄøĢīæÕ£©”£
A£®Éś³É¶žŃõ»ÆĢ¼ĘųĢåµÄÖŹĮæ
B£®°±»łĖįµÄĻą¶Ō·Ö×ÓÖŹĮæ
C£®ĶØČėŃõĘųµÄĢå»ż
D£®Éś³ÉĖ®µÄÖŹĮæ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com