
| ������ | Fe3+ | Fe2+ | Cu2+ |
| ��ʼ���� | 1.5 | 6.4 | 4.2 |
| ��ȫ���� | 3.2 | 8.9 | 6.7 |
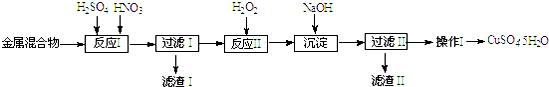
���� ��Cu��Fe������Au��Pt�Ƚ����Ļ�����������������ܽ�ͭ��������Ͳ����ܽ⣬���˵õ�����ΪAu��Pt����Һ�м����������������������Ϊ�����ӣ���������������Һ������Һ��PH��3.2--4.2��Χ�ڣ����������ӣ����˵õ�������Ϊ������������ҺΪ����ͭ��Һ��ͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ��������壬
��1�������Ļ�����м�����������ᣬCu��Fe�ᷢ����Ӧ��Ϊ��Һ��Au��Pt���ܽ⣻
��2����Ӧ���м���H2O2�������ǽ���Һ��Fe2+����ΪFe3+�����ڽ�����FeԪ�س�ȥ��
��3�����ɳ�����Ӧ��������������������������
��4���������Ǵ���Һ�еõ����ʾ��壬����IJ����Ǽ���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��5��2CuSO4•5H2O��2Cu2+��2I����I2��2S2O32-��n��S2O32-��=0.1mol/L��0.012L��10=0.012mol������õ�����ͭ�������ʵ���������õ���Ʒ�е������������������
��� �⣺��1����Cu��Fe������Au��Pt�Ƚ����Ļ�����м������������Cu��Fe�ᷢ����Ӧ��Ϊ��Һ��Au��Pt���ܽ⣬�������������Ҫ�ɷ���Au��Pt��
�ʴ�Ϊ��Au��Pt��
��2����Ӧ���м���H2O2�������ǽ���Һ��Fe2+����ΪFe3+��2Fe2++H2O2+2H+=2Fe3++2H2O��ͨ��������ҺPH���ڽ�����FeԪ�س�ȥ��
�ʴ�Ϊ��ʹFe2+����ΪFe3+��
��3�����ɳ�����Ӧ�����ӷ���ʽ�У�Fe3++3OH��=Fe��OH��3����
�ʴ�Ϊ��Fe3++3OH��=Fe��OH��3����
��4��������������ͭ��Һ�еõ�����ͭ���壬�����IJ����Ǽ���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ʴ�Ϊ������Ũ������ȴ�ᾧ��
��5��2Cu2++4I��=2CuI��+I2��I2+2S2O32-=2I��+S4O62-��
2CuSO4•5H2O��2Cu2+��2I����I2��2S2O32-��
n��S2O32-��=0.1mol/L��0.012L��10=0.012mol��
����n��CuSO4•5H2O��=0.012mol��
��������0.012mol��250g/mol=3.0g��
������Ʒ�е������������������$\frac{3.0g}{3.125g}$��100%=96%��
�ʴ�Ϊ��96%��
���� ���⿼��Ԫ�ؼ�����������ʡ����ʵij�ȥ�����ӷ���ʽ����д����ϵʽ���ڻ�ѧ�����е�Ӧ�ü����ʴ��ȵļ����֪ʶ�����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol H2SO4��������98 g/mol | |
| B�� | CO2��Ħ����������CO2����Է������� | |
| C�� | Ħ���������������ʵ����ʵ��������ʵ�����֮�����ϵ | |
| D�� | 1 mol�κ����ʵ��������ڸ����ʵ���Է������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ⶨ���� | ����Һ���/mL | ���������/mL | |
| �ζ�ǰ����/mL | �ζ������/mL | ||
| ��һ�� | 25.00 | 0.40 | 20.38 |
| �ڶ��� | 25.00 | 4.00 | 24.02 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1����
��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1����| �ζ�����ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.95 | 15.00 | 15.05 | 14.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
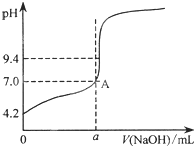 �����ڱ���������ر���Һ���ⶨNaOH��Һ��Ũ�ȣ�������������Һ���ζ��ڱ���������Һʱ�����в�����
�����ڱ���������ر���Һ���ⶨNaOH��Һ��Ũ�ȣ�������������Һ���ζ��ڱ���������Һʱ�����в�����| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.10 | 0.30 | 0.00 | 0.20 |
| V��NaOH��/mL���ն����� | 20.08 | 20.30 | 20.80 | 20.22 |
| V��NaOH��/mL�����ģ� | 19.98 | 20.00 | 20.80 | 20.02 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| n/mol T/�� | ����̿ | NO | E | F |
| ��ʼ | 2.030 | 0.100 | 0 | 0 |
| T1 | 2.000 | 0.040 | 0.030 | 0.030 |
| T2 | 2.005 | 0.050 | 0.025 | 0.025 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
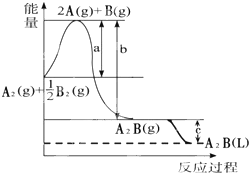 ��֪��A2��g��+$\frac{1}{2}$B2��g���TA2B��g������Ӧ�����������仯��ͼ���ʣ�
��֪��A2��g��+$\frac{1}{2}$B2��g���TA2B��g������Ӧ�����������仯��ͼ���ʣ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com