
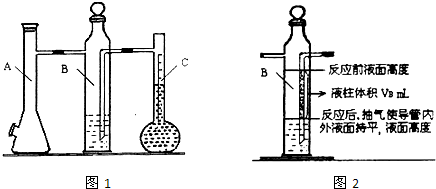
ijͬѧ����������ⶨ������þ�����ᷴӦ�ⶨ1 mol������������������գ�

��1��A�з�����Ӧ�����ӷ���ʽΪ______________________��
��2�����װ�������Եķ���������Bƿ�IJ�����������Ƥ������Aƿ���Ͽڣ�������__________________����ʱ������ȷ��װ�����������á�
��3����֪Һ����ƿ�Ŀ̶ȷ�Χ��110~130 mL��ʵ��ʱ��ȡþ��������Ҫ������0.100~0.110 g֮�䣬Ŀ����_________��
��4�����һ�βⶨʵ�飬��Ҫ2����ע����������������Ҫ��¼���ǵ�__________�γ������������
��5����������ᵼ��ʵ����ƫ�ߵ���______�����ţ�
a. þ���������Ĥû�г��� b. ��Һƿ�е�Һ����ˮ
c. δ��ȴ�����¾Ͷ��� d. װ�������Բ���
��1��Mg+2H+��Mg2++ H2����2�֣��� ��2��B�е�����Һ������������һ�����ڲ��½�����3��ȷ��������������ڿ̶ȷ�Χ֮�ڡ� ��4��2�� ��5��c��
��������
����������������Ʒֱ�Ϊ��A���巢������B��Һƿ��CҺ����ƿ��
��1��A����þ�����ᷴӦ��������þ�����������ݲ���д�����ӷ���ʽ��
��2��װ�������Կ�������װ��������ѹǿ�仯��Һ��仯�����ж�װ�������ԣ�
��3��ȡ110��30 mL��������ֵ������������������ʵ��������������ʵ�����þ�����ʵ�����ȣ������þ���������ͻ�õ���Χ0.100~0.110 g����ô����Ŀ�ĵ�Ȼ��ȷ��������������ڿ̶ȷ�Χ֮�ڡ�
��4��ÿ����ʵ��ʱ����ע������Aƿ���Ͽڳ�����ʹBƿ������Һ���ƽ��Bƿ������ѹǿ��������ѹ��ȣ���ע��ע�����γ�ʱҪ����ͷ�γ�����ʱ����ⶨ��ʼ״̬���ٴγ�����ѹ����ע������Aƿ���Ͽڴ�������ʹBƿ��Һ���ƽ������ʼ״̬��ͬ������ע�����г��������������¼��ֵ������ÿ������Ҫ��2��ע����������ƽ����3��ʵ�鹲��ע��������6�Ρ�
��5��a. þ�������������û�г�����������������ᷴӦ��û���������ɣ��ᵼ���������ƫС���ʴ���
b. ��Һƿ�е�Һ����ˮ���ᵼ���������ƫС���ʴ���
c.δ��ȴ�����¾Ͷ��������������������������ƫ����ȷ��
d. װ�������Բ��ã���Ȼ�ᵼ���������ƫС���ʴ���
���㣺����ʵ����Ʋⶨ������������ķ����Ͳ��衣


 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
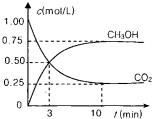 ��2013?��������ģ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
��2013?��������ģ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
| ||
| ||
| �ܽ�ȣ�S��/g | �ܶȻ���Ksp�� | ||
| Ca��OH��2 | Ba��OH��2 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
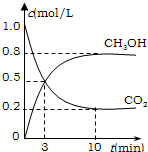 Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���| 1 |
| 2 |
| �ܽ�ȣ�S��/g | �ܶȻ���Ksp�� | ||
| Ca��OH��2 | Ba��OH��2 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijͬѧ����������ⶨ������þ�����ᷴӦ�ⶨ1mol������������������գ�
ijͬѧ����������ⶨ������þ�����ᷴӦ�ⶨ1mol������������������գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ����� | m��Mg��/g | �������/mL | Һ����ƿ��Һ�����/mL | ����������/mL | �������/mL | ����1mol�����/L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | X | |
| 2 | 0.115 | 10.0 | 121.0 | 8.0 |
| ʵ����� | m��Mg�� g |
�������mL | Һ����ƿ��Һ�����mL | ����������mL | Bƿ��һ��Һ�����mL | ˮ������ٷֺ��� | ����1mol�����L |
| 1 | 0.100 | 10.0 | 110.0 | 6.5 | VB | a% |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com