
 ��
������ ��1������Ԫ�ط��ţ��ж�Ԫ��ԭ�ӵĺ�����������ٸ��ݺ�������Ų�������д��
��2��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��3��VSEPRģ��Ϊ�ռ乹��Ϊ��������������ԭ�ӵļ۲���Ӷ�����4������sp3�ӻ�������ԭ�ӵļ۲���ӶԵ���Ŀ��4���ݴ��жϣ�
��4��1��N2�������2�����
��5��O���⻯��Ϊˮ��ˮ����֮�京����������˷��Ӽ���������
��6��������ͭ����μ��백ˮ�Ȳ���������ͭ������������ܽ�����[Cu��NH3��4]2+����Һ������ɫ��Һ��
��� ����𡿽⣺��1��CuԪ��Ϊ29��Ԫ�أ�ԭ�Ӻ�����29�����ӣ����Ժ�������Ų�ʽΪ��1s22s22p63s23p63d104s1��Cu2+�ļ۲��������9��������Cu2+�ļ۵����Ų�ͼΪ ��
��
�ʴ�Ϊ��1s22s22p63s23p63d104s1�� ��
��
��2��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����VA��Ԫ��Nԭ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ����Ե�һ�����ܴ�С˳����N��O��C���ʴ�Ϊ��N��O��C��
��3��CO32-��Cԭ�ӵļ۲���Ӷ�Ϊ$\frac{4+2}{2}$=3��VSEPRģ��Ϊƽ�������Σ�����ԭ�ӵ��ӻ���ʽΪsp2�ӻ���SO42-��Cԭ�ӵļ۲���Ӷ�Ϊ$\frac{4+2}{2}$=3��VSEPRģ��Ϊ�������壬�ʴ�Ϊ��sp2���������壻
��4��N2���ӽṹʽΪN��N��1��N2�����к���1���ȼ���2���м�������1molN2�к��Цм�����ĿΪ2NA���ʴ�Ϊ��2��
��5��O���⻯��Ϊˮ��ˮ����֮�京������⻯��ķе������ͬ��������Ԫ���⻯��ķе㣬�ʴ�Ϊ��ˮ����֮�京���ʹ��ˮ�ķе��������ķе㣻
��6��������ͭ����μ��백ˮ�Ȳ���������ͭ������������ܽ�����[Cu��NH3��4]2+����Һ������ɫ��Һ����Ӧ���ӷ���ʽΪ��Cu2++2NH3•H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ��Cu2++2NH3•H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
���� ������Ҫ������ԭ�ӵĵ����Ų������ӵĿռ乹�͡��縺�ԡ������������֪ʶ��ע��������������ʵ�Ӱ�죬�Ѷ��еȣ�


 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
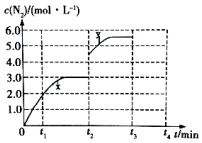 ��֪��298K��101kPa�����£������·�Ӧ��
��֪��298K��101kPa�����£������·�Ӧ��| ���ʣ�mol�� ʱ�� | NO | CO | N2 | CO2 |
| ��ʼ | 0.40 | 1.0 | ||
| 2min ĩ | 2.0 | 0.80 | 1.6 | |
| 4min ĩ | 1.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ĵ���ʽ��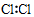 | B�� | �����Ľṹʽ��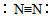 | ||
| C�� | Fһ�Ľṹʾ��ͼ�� | D�� | H2O2�ĵ���ʽ��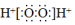 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�2.24 LCCl4�к�Clԭ����ĿΪ0.4NA | |
| B�� | 28g����ϩ������ԭ����Ϊ6NA | |
| C�� | 0.1mol����ϩ�к���˫������ĿΪ0.4NA | |
| D�� | ���³�ѹ�£�10 g 46%�ƾ�ˮ��Һ�к���ԭ������Ϊ0.1NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �縺�ԣ��ܣ��ۣ��ڣ��� | B�� | ԭ�Ӱ뾶���ܣ��ۣ��ڣ��� | ||
| C�� | ��һ�����ܣ��ܣ��ۣ��ڣ��� | D�� | ��������ϼۣ��ܣ��ۣ��ڣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SrΪ�ڵ�4���ڢ�A �� | |
| B�� | ${\;}_{38}^{90}$Sr������������������֮��Ϊ14 | |
| C�� | ${\;}_{38}^{90}$Sr��${\;}_{39}^{90}$Y��Ϊͬλ�� | |
| D�� | ${\;}_{38}^{90}$Sr�ĺ���������Ϊ38��SrԪ�ص����ԭ������Ϊ90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʵ���ҴӺ�����ȡ���ʵ�ķ����ǣ�ȡ�������ա��ܽ�����ˡ���ȡ | |
| B�� | ���Ҵ���Ũ�����Ʊ���ϩʱ������ˮԡ���ȿ��Ʒ�Ӧ���¶� | |
| C�� | �ñ���ʳ��ˮ���ˮ����ʯ��Ӧ�����Լ�����Ȳ�IJ������� | |
| D�� | ��������������ʴʱ������ʴ������������Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
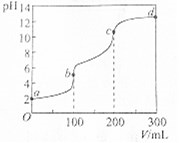 �����£���100mLһ��Ũ�ȵIJ��ᣨH2C2O4����Һ�м���0.1mol•L-1 NaOH��Һ��pH��NaOH��Һ����ı仯������ͼ��ʾ�������й�˵����ȷ���ǣ�������
�����£���100mLһ��Ũ�ȵIJ��ᣨH2C2O4����Һ�м���0.1mol•L-1 NaOH��Һ��pH��NaOH��Һ����ı仯������ͼ��ʾ�������й�˵����ȷ���ǣ�������| A�� | a����Һ��pH=2��������Һ��Ũ��Ϊ0.005mol•L-1 | |
| B�� | b���Ӧ��Һ�У�c��Na+����c��HC2O4-����c��OH-����c��C2O42-�� | |
| C�� | b��c�Σ���Ӧ�����ӷ���ʽΪHC2O4-+OH-�TC2O42-+H2O | |
| D�� | c��d�Σ���Һ��C2O42-��ˮ��̶�����ǿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com