
��16�֣�. ��ij���塢���Ļ�ɫ��Һ�У����ܺ���NH4+��Fe3+��Ba2+��Al3+��SO42-��HCO3-��I- Cl-���ӡ���������ʵ�飨�����Լ�����������
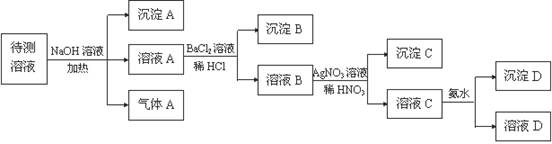
(1) ���� A�Ļ�ѧʽ�� ������A�Ļ�ѧʽ�� ��
(2)����Һ��һ������ ��һ�������� _______ �����ӷ���ʽ��ʾ��������һ�������ڵ�ԭ�� ��
(3)д����ҺC�������ˮ��Ӧ�����ӷ���ʽ ��
(4)������A�� ����A�� ����D�����ʵ�����Ϊ1mol,��SO42-�����ʵ���Ϊ�� mol
��1��Fe(OH)3 (1��) NH3��1�֣���2��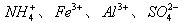 ��2�֣�
��2�֣�
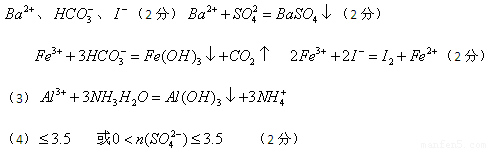
����������Һ�Ի�ɫ������һ�����������ӡ������Ӿ��������ԣ����������������ɵ��ʵ⡣ͬʱҲ�ܺ�HCO3���ˮ����������������CO2�����Ծ�һ�����ܺ���HCO3-��I-����������������������ɳ��������Ըó���һ������������������Ӧ���ǰ��������һ������NH4������ҺA�ܺ������ữ���Ȼ�����Ӧ���ɰ�ɫ���������Ըó��������ᱵ�����һ������SO42�������һ��û��Ba2������ҺB�к��������ӣ����ɳ���C���Ȼ�����������ǰ���������ữ�����Բ���ȷ���Ƿ��������ӡ���ҺC�ܺ����İ�ˮ��Ӧ���ɳ��������Ըó���ֻ����������������ԭ��Һ�л�����Al3����������A�� ����A�� ����D�����ʵ�����Ϊ1mol,����Һ�������ӵĵ������7mol�����ڲ����ų������ӣ�����SO42�������ʵ���Ӧ����С�ڵ���3.5mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ������һ�и���9���¿������ۺϻ�ѧ�Ծ����������� ���ͣ������
��16�֣�. ��ij���塢���Ļ�ɫ��Һ�У����ܺ���NH4+��Fe3+��Ba2+��Al3+��SO42-��HCO3-��I- Cl-���ӡ���������ʵ�飨�����Լ�����������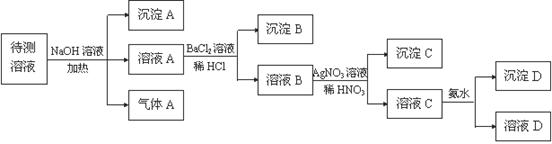
(1) ���� A�Ļ�ѧʽ�� ������A�Ļ�ѧʽ�� ��
(2)����Һ��һ������ ��һ�������� _______ �����ӷ���ʽ��ʾ��������һ�������ڵ�ԭ�� ��
(3)д����ҺC�������ˮ��Ӧ�����ӷ���ʽ ��
(4)������A������A������D�����ʵ�����Ϊ1mol,��SO42-�����ʵ���Ϊ�� mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ������һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
(10��)��ij���塢����dz��ɫ��Һ�У����ܺ���K+��NH4+��Fe3+��Ba2+��Al3+��SO42-��HCO3-��Cl-���ӡ���������ʵ�飨�����Լ�����������

(1)����A�Ļ�ѧʽ�� ������A�Ļ�ѧʽ�� ��
(2)����Һ��һ������ ��һ�������� _______ ��
(3)д����ҺC�������ˮ��Ӧ�����ӷ���ʽ ��
(4)������þ����������θ������θ��������Ϊ�����ʲ�����ˮ�����ú����к�θ�ᣬ���ó־á���������þ(Mg2Si3O8��nH2O)��д�����������ʽΪ ��
���к�θ��(HCl)�����ӷ���ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ����9���¿���ѧ���� ���ͣ�ѡ����
��ij���塢����dz��ɫ��Һ�У����ܺ������а������ӣ�H����NH��Fe3����Ba2����Al3����SO��HCO��I�����ڼ��鷽�����ʱ������������Һ�����ɺ����ӣ�������OH������ �� ��
A��4�� B��5�� C��6�� D��7��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com