
�Իش��������⣺
��1����֪24��A��40��Bǡ����ȫ��Ӧ����0��8molC��32��D����C��Ħ������Ϊ ��
��2����ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
�ٸ�Ũ������HCl�����ʵ���Ũ��Ϊ mol/L��
����ʵ��������450mL2��38 mol/L��ϡ���ᣬ���ø�Ũ����________ mL,����ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������______________________(����������)��
��ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________2��38mol/L (����ڡ������ڡ���С�ڡ�����ͬ)��
��1��40g��mol_1 (û��λ��0��)
��2�� ��11��9 ��100 A��C(ȫ�Բŵ÷�)���ձ���������(ȫд�ŵ÷�)�� С��
���������������1�����������غ㶨�ɣ�C������Ϊ��24g+40g-32g=32g����C��Ħ������Ϊ��32g��0��8mol=40g/mol��
��2��������������Ϊ1L�����Ũ����HCl�����ʵ���Ũ��Ϊ��1000��1��19��36��5%��36��5mol?L?1 =11��9mol?L?1��
����Ϊ����ƿ���û��450mL������Ӧѡ��500mL����ƿ��������Ũ��������ΪVL����0��5L��2��38mol?L?1=V��11��9mol?L?1���ɵ�V=0��1L=100mL��ȡŨ�����õ���Ͳ��������Һ�õ�500mL����ƿ������ʱ�õ���ͷ�ιܣ����Բ���ҪA��ƿ��C��Һ©����ϡ��Ũ���ỹ��Ҫ�ձ��Ͳ����������ֲ�������������ʱ���ӿ̶��ߣ���ʹ��Һ����������������Һ��Ũ��С��2��38mol?L?1��
���㣺���⿼�������غ㶨�ɵ�Ӧ�á����ʵ���Ũ�ȵļ��㡢һ�����ʵ���Ũ�ȵ���Һ�����ơ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijУ��ѧС��ѧ�����С�������Է��������IJⶨ����ʵ�顣�������£����������ݻ�����ȵ���ƿ�ռ����壬�����ռ����������ƿ���������ݼ��±�(�ѻ���ɱ�״���µ���ֵ)��
| ���� | ��ƿ�������������(g) |
| A | 48.4082 |
| B | 48.4082 |
| C | 48.4082 |
| D | 48.4342 |
| E | 48.8762 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ������һƿ�����ơ�84����Һ��������������Ϻ�����Һ��װ˵���õ�������Ϣ��
��84����Һ������25%NaClO 1 000 mL���ܶ�1.19 g��cm��3��ϡ��100��(�����)��ʹ�á�
�����������Ϣ�����֪ʶ�ش��������⣺
(1)�á�84����Һ�������ʵ���Ũ��Ϊ________ mol��L��1��
(2)��ͬѧȡ100 mL�á�84����Һ��ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na��)��________ mol��L��1(����ϡ�ͺ���Һ�ܶ�Ϊ1.0 g��cm��3)��
(3)ijʵ������480 mL��25%NaClO������Һ����ͬѧ���ĸá�84����Һ�����䷽������NaClO�������Ƹ�����Һ��
������˵����ȷ����________��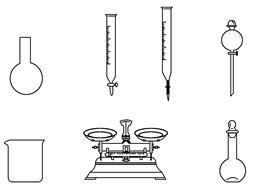
A������ͼ��ʾ�������У��������Dz���Ҫ�ģ�����һ�ֲ�������
B������ƿ������ˮϴ����Ӧ��ɲ���������Һ����
C�����ù������ƷNaClO�����ƿ��ܵ��½��ƫ��
D����Ҫ������NaClO��������Ϊ143 g
�������ƹ����У����в�������ʹ���Ƶ���Һ��Ũ��ƫ�����________��
A���ձ�����Һת�Ƶ�����ƿ��ʱ��δϴ���ձ�
B������ʱ�����ӿ̶���
C������ʱ�����ӿ̶���
D����Һʱ��������Һ�彦��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�̷���FeSO4��7H2O���ڻ�ѧ�ϳ���������ԭ������������ҵ�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷���
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ ����>��<��=��40%��
��2��ʵ��������20%�������ᣨ100�˷������ẬSO3 20�ˣ�����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL����״��������ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200mL2mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS������������������λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10mLNa2CO3��Na2SO4�Ļ����Һ�м����������Ȼ�����Һ�����ɳ���������Ϊ6.27g�������ó����м�������ϡ����,�����������ٵ�2.33g,���ų�����,�Լ��㣺
��1��ԭ�������Na2SO4�����ʵ���Ũ��Ϊ mol?L-1��
��2���ڱ�״���²�����������Ϊ L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����ý�̿��ʯ��Ҥ��ȼ�շ��ȣ�ʹʯ��ʯ�ֽ�����CO2����Ҫ��Ӧ���£�
C+O2��CO2 �٣� CaCO3��CO2��+CaO ��
��1����̼���95%��ʯ��ʯ2.0 t������ȫ�ֽ⣨�����ʲ��ֽ⣩���ɵñ�״����CO2�����Ϊ_________________m3��
��2��������CaCO3�ͽ�̿���٢���ȫ��Ӧ����Ҥ�������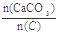 =2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
=2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
��3��ij��Ҥ���ɷ����£�O2 0.2%��CO 0.2%��CO2 41.6%������ΪN2����˴�Ҥ������� Ϊ��ֵ��
Ϊ��ֵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ��������480 mL 0��1mol·L-1 NaOH��Һ���ش���������
��1��Ӧ��������ƽ��ȡ�������ƹ��� g��
��2������NaOH��Һʱ���õ���Ҫ������������ƽ��ҩ�ס��ձ�������������Ͳ �� ��
ʹ������ƿǰ������еIJ����� ��
��3������ʱ����ʵ�������õ��������������÷ֱ��� �� ��
��4������ʱ����ˮ�����̶��ߣ�Ӧ��δ����� ��
��5���Է������в�����������Һ��Ũ���к�Ӱ�졣
A�� ƫ�� B�� ƫ�� C�����䣨�÷��Żش�
�� ����ʱ���ӿ̶��� ��
�� ������ֽ�ϳ���NaOH���� ��
������ƿû�и������������ˮ�� ��
�ܶ��ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ� ��
��6������������Լ�ƿ��ʢ���������ƺõ���Һ���ϱ�ǩ����������ȥ (��ǩ��ͼ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����ag�Ȼ�������1.8Lˮ�У�ǡ��ʹ����������ˮ������֮��Ϊ1:100����aֵΪ ��
��2���ڷ�Ӧ2A+B=3C+2D�У���֪3.4gA��3.2gB��ȫ��Ӧ������4.8gC����֪��D��ʽ��Ϊ18����B��ʽ����
��3��25.4g ij���۽����Ȼ���(ACl2)�к���0.4mol Cl������ACl2��Ħ�������� ��A�����ԭ�������� ��ACl2�Ļ�ѧʽ�� ��
��4�� ij�������Na2SO4��Al2��SO4��3��ɣ���֪Na��Al��Ԫ�ص�����֮��Ϊ23: 9����Na2SO4��Al2��SO4��3���ʵ���֮��Ϊ ����1.00mol SO42�C�ĸû���������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ������NaOH��������250mL 1��25mol/L��NaOH��Һ����ղ���ش��������⣺
��1������ʱ����IJ��������У��ձ����������� �� ��
��2������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������ƽȷ��ȡ�����NaOH����������������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
C��������ȴ��NaOH��Һ�ز�����ע��250mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��3���������Ƶ���ҺŨ��ƫ�͵��� ��
A������NaOHʱ���������������
B��������ƿ��ת����Һʱ(ʵ�鲽��C)������Һ����������ƿ����
C��������ˮʱ���������˿̶���
D������ʱ���ӿ̶���
E������ǰ������ƿ������������ˮ
��4��ijͬѧ���ù���Na2CO3����Na2CO3��Һ�Ĺ�����ͼ��ʾ��������������� 

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com