
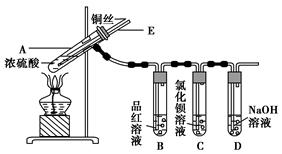
| µĪ¼ÓµÄČÜŅŗ | ĀČĖ® | °±Ė® |
| ³ĮµķµÄ»ÆѧŹ½ | | |
 CuSO4£«SO2”ü£«2H2O
CuSO4£«SO2”ü£«2H2O CuSO4£«SO2”ü£«2H2O”£(2)ĄūÓĆSO2ÄÜŹ¹Ę·ŗģČÜŅŗĶĖÉ«µÄŠŌÖŹĄ“Ö¤Ć÷CuÓėÅØĮņĖį·“Ó¦²śÉśĮĖSO2ĘųĢ唣(3)ĀČĖ®ÖŠµÄCl2¾ßÓŠŃõ»ÆŠŌ£¬Äܽ«SO2Ńõ»ÆĪŖSO42-£¬SO42-ÓėBa2£«·“Ӧɜ³ÉBaSO4³Įµķ£»°±Ė®¾ßÓŠ¼īŠŌ£¬ĪüŹÕSO2Éś³ÉSO32-£¬SO32-ÓėBa2£«·“Ӧɜ³ÉBaSO3³Įµķ”£(4)µ¼¹ÜEÓė“óĘųĻąĶØ£¬ÄÜŹ¹×°ÖĆÄŚµÄŃ¹Ēæŗć¶Ø£¬·ĄÖ¹³öĻÖµ¹ĪüĻÖĻó”£(5)²ŠĮōµÄÓŠ¶¾ĘųĢåĪŖSO2£¬Ö»ŅŖĄūÓĆæÕĘų½«SO2»ŗĀżøĻČėNaOHČÜŅŗÖŠ£¬Ź¹SO2±»ĶźČ«ĪüŹÕ¼“æÉ”£(6)SO2ÓėNa2S·“Ó¦£¬Ź×ĻČŹĒSO2ÓėH2O·“Ӧɜ³ÉH2SO3£ŗSO2£«H2O??H2SO3£¬H2SO3ÓėNa2S·“Ӧɜ³ÉH2S£ŗNa2S£«H2SO3=H2S”ü£«Na2SO3£¬SO2ÓėH2S·“Ӧɜ³ÉS£ŗ2H2S£«SO2=3S”ż£«2H2O£¬øĆ¹ż³ĢæɱķŹ¾ĪŖ3SO2£«2Na2S=3S”ż£«2Na2SO3£¬n mol Na2SĪüŹÕ1.5n mol SO2£¬Ķ¬Ź±Éś³Én mol Na2SO3£¬Na2SO3ĪüŹÕSO2Éś³ÉNaHSO3£ŗNa2SO3£«SO2£«H2O=2NaHSO3£¬øĆ²½·“Ó¦ÓÖæÉĪüŹÕn mol SO2£¬¹Ź×ī¶ąÄÜĪüŹÕ2.5n mol SO2”£
CuSO4£«SO2”ü£«2H2O”£(2)ĄūÓĆSO2ÄÜŹ¹Ę·ŗģČÜŅŗĶĖÉ«µÄŠŌÖŹĄ“Ö¤Ć÷CuÓėÅØĮņĖį·“Ó¦²śÉśĮĖSO2ĘųĢ唣(3)ĀČĖ®ÖŠµÄCl2¾ßÓŠŃõ»ÆŠŌ£¬Äܽ«SO2Ńõ»ÆĪŖSO42-£¬SO42-ÓėBa2£«·“Ӧɜ³ÉBaSO4³Įµķ£»°±Ė®¾ßÓŠ¼īŠŌ£¬ĪüŹÕSO2Éś³ÉSO32-£¬SO32-ÓėBa2£«·“Ӧɜ³ÉBaSO3³Įµķ”£(4)µ¼¹ÜEÓė“óĘųĻąĶØ£¬ÄÜŹ¹×°ÖĆÄŚµÄŃ¹Ēæŗć¶Ø£¬·ĄÖ¹³öĻÖµ¹ĪüĻÖĻó”£(5)²ŠĮōµÄÓŠ¶¾ĘųĢåĪŖSO2£¬Ö»ŅŖĄūÓĆæÕĘų½«SO2»ŗĀżøĻČėNaOHČÜŅŗÖŠ£¬Ź¹SO2±»ĶźČ«ĪüŹÕ¼“æÉ”£(6)SO2ÓėNa2S·“Ó¦£¬Ź×ĻČŹĒSO2ÓėH2O·“Ӧɜ³ÉH2SO3£ŗSO2£«H2O??H2SO3£¬H2SO3ÓėNa2S·“Ӧɜ³ÉH2S£ŗNa2S£«H2SO3=H2S”ü£«Na2SO3£¬SO2ÓėH2S·“Ӧɜ³ÉS£ŗ2H2S£«SO2=3S”ż£«2H2O£¬øĆ¹ż³ĢæɱķŹ¾ĪŖ3SO2£«2Na2S=3S”ż£«2Na2SO3£¬n mol Na2SĪüŹÕ1.5n mol SO2£¬Ķ¬Ź±Éś³Én mol Na2SO3£¬Na2SO3ĪüŹÕSO2Éś³ÉNaHSO3£ŗNa2SO3£«SO2£«H2O=2NaHSO3£¬øĆ²½·“Ó¦ÓÖæÉĪüŹÕn mol SO2£¬¹Ź×ī¶ąÄÜĪüŹÕ2.5n mol SO2”£


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
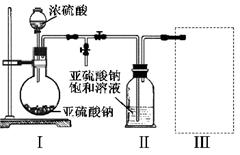
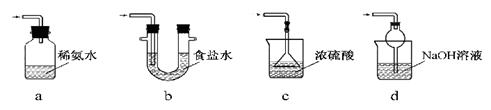

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 Fe2O3£«SO2”ü£«SO3”ü£¬Čō½«Éś³ÉµÄĘųĢåĶØČėĀČ»Æ±µČÜŅŗÖŠ£¬µĆµ½µÄ³ĮµķĪļŹĒ( )
Fe2O3£«SO2”ü£«SO3”ü£¬Čō½«Éś³ÉµÄĘųĢåĶØČėĀČ»Æ±µČÜŅŗÖŠ£¬µĆµ½µÄ³ĮµķĪļŹĒ( )| A£®BaSO3ŗĶBaSO4 | B£®BaS | C£®BaSO3 | D£®BaSO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
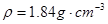 £© 60mL³ä·Ö·“Ó¦£¬ŠæČ«²æČܽā£¬¶ŌÓŚÖʵƵÄĘųĢ壬ӊĶ¬Ń§ČĻĪŖæÉÄÜ»ģÓŠŌÓÖŹ”£
£© 60mL³ä·Ö·“Ó¦£¬ŠæČ«²æČܽā£¬¶ŌÓŚÖʵƵÄĘųĢ壬ӊĶ¬Ń§ČĻĪŖæÉÄÜ»ģÓŠŌÓÖŹ”£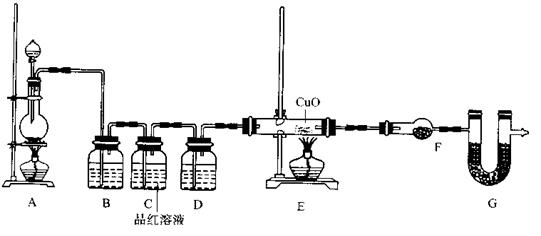
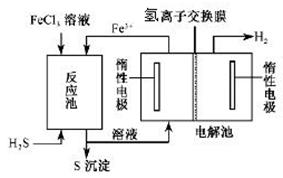
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĶÓėÅØĮņĖį·“Ó¦ĖłµĆ°×É«¹ĢĢå²»ŹĒCuSO4 |
| B£®¼ÓBaCl2ČÜŅŗŗóĖłµĆ°×É«³ĮµķŹĒBaSO3 |
| C£®°×É«¹ĢĢåÖŠ¼ŠŌÓµÄÉŁĮæŗŚÉ«ĪļÖŹæÉÄÜŹĒCuO |
| D£®°×É«¹ĢĢåÖŠ¼ŠŌÓµÄÉŁĮæŗŚÉ«ĪļÖŹÖŠŅ»¶Øŗ¬ÓŠŌŖĖŲCuŗĶS |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĀČĖ®ÖŠĶØČėŅ»¶ØĮ涞Ńõ»ÆĮņĘųĢåæÉŌöĒæĀČĖ®µÄĘư׊Ō |
| B£®ŗ¬ÓŠ·ÓĢŖµÄĒāŃõ»ÆÄĘČÜŅŗÖŠĶØČė¶žŃõ»ÆĮņĘųĢ壬ČÜŅŗ±äĪŽÉ«£¬ĖµĆ÷¶žŃõ»ÆĮņ¾ßÓŠĘư׊Ō |
| C£®¶žŃõ»ÆĮņ¼Čæɱ»Ńõ»ÆŅ²æɱ»»¹Ō |
| D£®×ĻÉ«ŹÆČļŹŌŅŗÖŠĶØČė¶žŃõ»ÆĮņ£¬ČÜŅŗĻȱäŗģŗóĶĖÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

| A£®Ė® | B£®±„ŗĶNaHSO3ČÜŅŗ | C£®ĖįŠŌKMnO4ČÜŅŗ | D£®NaOHČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
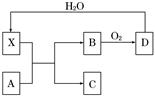
| A£®XŹ¹ÕįĢĒ±äŗŚµÄĻÖĻóÖ÷ŅŖĢåĻÖĮĖXµÄĒæŃõ»ÆŠŌ |
| B£®ČōAĪŖĢś£¬Ōņ×ćĮæAÓėXŌŚŹŅĪĀĻĀ¼“æÉĶźČ«·“Ó¦ |
| C£®ČōAĪŖĢ¼µ„ÖŹ£¬Ōņ½«CĶØČėÉŁĮæµÄ³ĪĒåŹÆ»ŅĖ®£¬Ņ»¶ØæÉŅŌ¹Ū²ģµ½°×É«³Įµķ²śÉś |
| D£®¹¤ŅµÉĻ£¬B×Ŗ»ÆĪŖDµÄ·“Ó¦Ģõ¼žĪŖøßĪĀ”¢³£Ń¹”¢Ź¹ÓĆ“ß»Æ¼Į |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĶÓėÅØĮņĖį¹²ČČ |
| B£®ÓĆĶʬĪŖŃō¼«£¬ŹÆÄ«ĪŖŅõ¼«£¬µē½āĻ”ĮņĖį |
| C£®ĻČ×ĘÉÕ·ĻĶŠ¼Éś³ÉŃõ»ÆĶ£¬Č»ŗóŌŁÓĆÅØĮņĖįČܽā |
| D£®ŹŹµ±ĪĀ¶ČĻĀ£¬Ź¹ĶʬŌŚ³ÖŠųĶØČėæÕĘųµÄĻ”ĮņĖįÖŠČܽā |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com