
��������������ظ���λ��Ϊ������NaCl����ṹ��ͼ��ʾ����֪ ���徧���ṹΪNaCl�ͣ����ھ���ȱ�ݣ�xȡֵС��1����֪
���徧���ṹΪNaCl�ͣ����ھ���ȱ�ݣ�xȡֵС��1����֪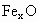 �����ܶ�Ϊ
�����ܶ�Ϊ �������߳�Ϊ4.28*10-10m
�������߳�Ϊ4.28*10-10m

(1) ��xֵ(��ȷ��0.01)Ϊ______��
��xֵ(��ȷ��0.01)Ϊ______��
(2)�����е�Fe�ֱ�Ϊ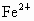 ��
�� ����
���� ��
�� ��������
�������У� ��ռ����(��С����ʾ����ȷ��0.001)Ϊ______��
��ռ����(��С����ʾ����ȷ��0.001)Ϊ______��
(3)�˾��廯ѧʽΪ_______________________��
(4)��ij�� (��
(�� )��������ҵȾ����
)��������ҵȾ���� Χ�ɵĿռ伸����״��______��
Χ�ɵĿռ伸����״��______��
(5)�ھ����У���Ԫ�ص����Ӽ���̵ľ���Ϊ______m��
|
(1)x=0.92 ��(2)0.826��(3)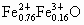 ��(4)�������塡(5) ��(4)�������塡(5)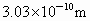
˼·������ (1)��NaCl����ṹ��֪��1��NaCl������8��С�����幹�ɣ�ÿ��С�������8������ֱ���4��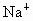 ��4�� ��4��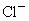 ����ռ�ݣ�ÿ�� ����ռ�ݣ�ÿ��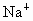 �� ��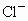 ��ֻ��1/8ռ����С�������У����ÿ��С�����庬 ��ֻ��1/8ռ����С�������У����ÿ��С�����庬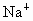 ��Ϊ(1/8)��4=1/2(��)���� ��Ϊ(1/8)��4=1/2(��)����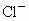 ��Ϊ(1/8)��4=1/2(��)������NaCl������8��С��������ɣ�ÿ��������NaCl����Ϊ8��(1/2)=4(��)��ͬ������ ��Ϊ(1/8)��4=1/2(��)������NaCl������8��С��������ɣ�ÿ��������NaCl����Ϊ8��(1/2)=4(��)��ͬ������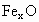 ��������4�� ��������4��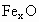 ������1mol��������4 mol ������1mol��������4 mol 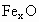 ���� ����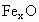 ��Ħ������ΪM������ ��Ħ������ΪM������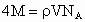 �� ��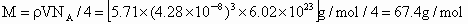 ����x=0.92�� ����x=0.92��
(2) �躬y��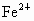 ������(0.92��y)�� ������(0.92��y)��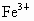 �������������ϼ۴�����Ϊ�㣬�ã�2y��3(0.92��y)=2�����y=0.76(��)���� �������������ϼ۴�����Ϊ�㣬�ã�2y��3(0.92��y)=2�����y=0.76(��)����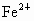 ��ռ����Ϊ0.76/0.92=0.826�� ��ռ����Ϊ0.76/0.92=0.826��
(3) ����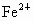 Ϊ0.76���� Ϊ0.76����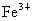 Ϊ(0.92��0.76)=0.16���ʻ�ѧʽΪ Ϊ(0.92��0.76)=0.16���ʻ�ѧʽΪ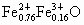 �� ��
(4) ��NaCl����ṹ����֪����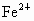 (�� (��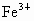 )��������ҵȾ���� )��������ҵȾ����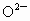 ��6������6�� ��6������6��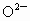 ��Χ�ɵļ�����״Ϊ�������壮 ��Χ�ɵļ�����״Ϊ�������壮
(5) ��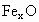 ��Fe��Fe��̵ľ���Ϊr���� ��Fe��Fe��̵ľ���Ϊr���� �����һ��ƽ��ͼ(��ͼ��ʾ)��֪����ֱ��������ABC�У�б��BC����Ϊ�ã���AB=AC=�����߳���1/2�� �����һ��ƽ��ͼ(��ͼ��ʾ)��֪����ֱ��������ABC�У�б��BC����Ϊ�ã���AB=AC=�����߳���1/2��
��� 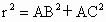 �� ��
���ɼ����ܽ������Ĺؼ�������������⽫ 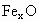 ���徧���Ľṹת��Ϊ��Ϥ��NaCl�����ṹ�������� ���徧���Ľṹת��Ϊ��Ϥ��NaCl�����ṹ��������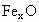 ��xֵʵ������ ��xֵʵ������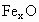 ��ʽ����Ħ�������������ܶȺ;����߳��������4�� ��ʽ����Ħ�������������ܶȺ;����߳��������4��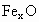 ��ʽ�������þ���ĵ����Կ���� ��ʽ�������þ���ĵ����Կ����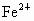 �� ��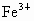 �ĸ����ȣ��������� �ĸ����ȣ���������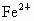 ��ռ�ķ����������У���Ԫ��֮����̾��룬��Ϊһ��С������Խ��ߵij��ȣ� ��ռ�ķ����������У���Ԫ��֮����̾��룬��Ϊһ��С������Խ��ߵij��ȣ� |


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)Aͼ��ij���ӻ�����ľ���(��ɾ����һ����С�ظ���λ)��������λ���м䣬������λ��8�����㣬�û����������������ӵĸ�������_______________��

(2)Bͼ��ʾ����NaCl�����һ��������ͨ����������������ȷ��һ��NaCl�����к�Na+��Cl-�ĸ����ֱ�Ϊ_______________��_______________��
(3)Cͼ�ǽ��ʯ�ľ���ṹ��C60�����ʯ��ʯī���ߵĹ�ϵ��_______________��
A.ͬ���칹�� B.ͬ�������� C.ͬϵ�� D.ͬλ��
�辧��Ľṹ�����ʯ���ƣ�1 mol�辧���к��й�赥������ĿԼ��_______________NA������������Ľṹ�൱���ڹ辧��ṹ��ÿ���衪�赥��֮�����һ����ԭ�ӡ���������Ŀռ���״�ṹ�У��衢��ԭ���γɵ���С����ԭ����Ŀ��_______________��
(4)ʯī����ṹ��Dͼ��ʾ��ÿһ�����������������ι��ɣ���ƽ��ÿһ������������ռ�е�̼ԭ����Ϊ_______________����C��C������Ϊ_______________��
(5)����ϩC60�ṹ����������Eͼ����C60����_______________�������Σ�_______________������Ρ���̬ʱ��C60����_______________ (����ӡ�����ԭ�ӡ����ӡ�)���壬C60�����к���˫������Ŀ��_______________��
(6)������Ļ����ṹ��Ԫ��������ԭ����ɵ�����ʮ�����ԭ�Ӿ��壬��Fͼ�����к���20���ȱ������κ�һ����Ŀ�Ķ��ǣ�ÿ�������ϸ���1��ԭ�ӡ��Թ۲�Fͼ���ƶ���������ṹ��Ԫ������ԭ�Ӹ��������ǡ���B��B�����ĸ�������Ϊ______________��_______________��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ�ش����⣺
(1)Aͼ��ij���ӻ�����ľ���(��ɾ����һ����С�ظ���λ)��������λ���м䣬����
��λ��8�����㣬�û����������������ӵĸ�������_______________��

(2)Bͼ��ʾ����NaCl�����һ��������ͨ����������������ȷ��һ��NaCl�����к�Na+
��Cl-�ĸ����ֱ�Ϊ_______________��_______________��
(3)Cͼ�ǽ��ʯ�ľ���ṹ��C60�����ʯ��ʯī���ߵĹ�ϵ��_______________��
A.ͬ���칹�� B.ͬ�������� C.ͬϵ�� D.ͬλ��
�辧��Ľṹ�����ʯ���ƣ�1 mol�辧���к��й�赥������ĿԼ��_______________NA������������Ľṹ�൱���ڹ辧��ṹ��ÿ���衪�赥��֮�����һ����ԭ�ӡ���������Ŀռ���״�ṹ�У��衢��ԭ���γɵ���С����ԭ����Ŀ��_______________��
(4)ʯī����ṹ��Dͼ��ʾ��ÿһ�����������������ι��ɣ���ƽ��ÿһ������������ռ�е�̼ԭ����Ϊ_______________����C��C������Ϊ_______________��
(5)����ϩC60�ṹ����������Eͼ����C60����_______________�������Σ�_______________������Ρ���̬ʱ��C60����_______________ (����ӡ�����ԭ�ӡ����ӡ�)���壬C60�����к���˫������Ŀ��_______________��
(6)������Ļ����ṹ��Ԫ��������ԭ����ɵ�����ʮ�����ԭ�Ӿ��壬��Fͼ�����к���
20���ȱ������κ�һ����Ŀ�Ķ��ǣ�ÿ�������ϸ���1��ԭ�ӡ��Թ۲�Fͼ���ƶ���������ṹ��Ԫ������ԭ�Ӹ��������ǡ���B��B�����ĸ�������Ϊ______________��_______________��_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com