
| A�� | Ҫ����Ũ��Ϊ0.25mol•L-1��NaOH��Һ480mL��Ӧ����4.8g NaOH��250mL���ձ����ܽ⣬��ȴ����ת�Ƶ�500mL����ƿ�У�ϴ�ӡ�ת�ơ����� | |
| B�� | ����NaOH��Һ�����ձ����ܽ�NaOH��δ��ȴ�����¾�ת�Ƶ�����ƿ�У���ҺŨ��ƫ�� | |
| C�� | ����һ�����ʵ���Ũ�ȵ���Һ����ʱ�����ӿ̶��ߵ�������Ũ��ƫ�� | |
| D�� | ����20g�ܶ�Ϊ��g•cm-3��Ca��NO3��2��Һ�к���2g Ca��NO3��2������Һ��NO3-�����ʵ���Ũ��Ϊ25��/41mol•L-1 |
���� A������m=CVM������Ҫ�����������Ƶ�������
B�����������ܽ���ȣ�δ��ȴ�����¾�ת�Ƶ�����ƿ�У���ȴ����Һ���ƫС������C=$\frac{n}{V}$������������
C������ʱ�����ӿ̶��ߵ���������Һ���ƫС������C=$\frac{n}{V}$������������
D������V=$\frac{m}{��}$������������Һ��������ٸ���n=$\frac{m}{M}$�����4g����Ƶ����ʵ������������ƵĻ�ѧʽ�������Һ����������ӵ����ʵ�����������c��NO3-��=$\frac{n}{V}$�������������ӵ����ʵ���Ũ�ȣ�
��� �⣺A������Ũ��Ϊ0.25mol•L-1��NaOH��Һ480mL��ʵ����û��480mL������Ӧѡ��500mL����ƿ��ʵ������500mL��Һ����Ҫ���ʵ�����m=0.25mol/L��0.5L��40g/mol=5.0g����A����
B�����������ܽ���ȣ�δ��ȴ�����¾�ת�Ƶ�����ƿ�У���ȴ����Һ���ƫС������c=$\frac{n}{V}$����ҺŨ��ƫ�ߣ���B����
C������ʱ�����ӿ̶��ߵ���������Һ���ƫС������c=$\frac{n}{V}$����ҺŨ��ƫ�ߣ���C��ȷ��
D�����������Һ�����V=$\frac{20g}{��g/mL}$=$\frac{20}{��}$mL��2g����Ƶ����ʵ���Ϊ$\frac{2g}{164g/mol}$=$\frac{1}{82}$mol������������Һ�к�����������ӵ����ʵ���Ϊ$\frac{1}{82}$��2=$\frac{1}{41}$mol�������������Ũ��Ϊ$\frac{\frac{\frac{1}{41}}{20}}{�ѡ�1{0}^{-3}}$=$\frac{50��}{41}$mol•L-1����D����
��ѡ��C��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ����й����ʵ���Ũ�ȵļ��㣬��ȷ���ʵ���Ũ�ȸ����ǽ���ؼ���ע������c=$\frac{n}{V}$�����������ķ�������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.5 mol | B�� | 1.0 mol | C�� | 2.0 mol | D�� | 4.0 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ԫ�صķǽ����ԣ�X��W��Z | |
| B�� | Ԫ��W����ϼۺ���ͻ��ϼ۵Ĵ�����Ϊ0 | |
| C�� | Y��X���γ����ӻ����� | |
| D�� | ԭ�Ӱ뾶��Z��Y��X��W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
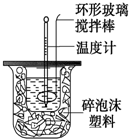 ������ͼװ�òⶨ�кͷ�Ӧ�ķ�Ӧ�ȵ�ʵ�鲽�����£�
������ͼװ�òⶨ�кͷ�Ӧ�ķ�Ӧ�ȵ�ʵ�鲽�����£�| �¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 3 | 26.4 | 26.2 | 26.3 | 29.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ϊʹ�������ʼӿ죬���ò������ڹ�������������裬����Һ������ | |
| B�� | �þƾ��Ƹ��Թܼ���ʱ��Ҫ�������ȵ��Թܷ��ھƾ��ƻ���������� | |
| C�� | Ϊ���ٹ������ʵ��ܽ�ֻ�ܲ��ü��ȵķ��� | |
| D�� | Ϊ�����������ʵ��ܽ�ȣ�����ȡ���衢���ȵȴ�ʩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢܢ� | B�� | �ݢޢߢ� | C�� | �٢ݢޢߢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com