
·ÖĪö ³£ĪĀĻĀ0.01mol•L-1 H2SO4£ØĒæĖį£©ČÜŅŗÖŠc£ØH+£©=0.011mol•L-1£¬ĖµĆ÷ĮņĖįµÄµŚ¶ž²½µēĄė“ęŌŚµēĄėĘ½ŗā£¬
£Ø1£©ĮņĖįČÜŅŗÖŠĶźČ«µēĄė£¬ĮņĖįĒāøłĄė×ӵĵēĄė³Ģ¶Č“óÓŚĘäĖ®½ā³Ģ¶Č£¬ČÜŅŗĻŌŹ¾ĖįŠŌ£¬¾Ż“ĖÅŠ¶Ļø÷Ąė×ÓÅØ¶Č“óŠ”£»ĮņĖįÄĘČÜŅŗÖŠ£¬ĮņĖįøłĄė×Ó²æ·ÖĖ®½āÉś³ÉĮņĖįĒāøłĄė×Ó£¬ČÜŅŗĻŌŹ¾¼īŠŌ£¬¾Ż“ĖŠ“³öĘäĖ®½ā·½³ĢŹ½£»
£Ø2£©ĮņĖįµÄµŚŅ»²½µēĄėŅÖÖĘĮĖĘ䵌¶ž²½µēĄė£¬µ¼ÖĀĮņĖįÖŠĮņĖįĒāøłĄė×ӵĵēĄė³Ģ¶Č¼õŠ”£¬¾Ż“ĖÅŠ¶Ļ0.01mol/LµÄĮņĖįĒāÄĘČÜŅŗÖŠĒāĄė×ÓÅØ¶Č“óŠ”£»
£Ø3£©ĮņĖįĒāÄĘÓėĒāŃõ»Æ±µ·“Ӧɜ³ÉĮņĖį±µŗĶĖ®£¬ĘäÖŠĮņĖįĒāÄĘ²»ÄܲšæŖ£¬ĮņĖįøłĄė×Ó¹żĮ棻¼ĢŠųµĪ¼ÓĒāŃõ»Æ±µ£¬Ź£ÓąµÄĮņĖįøłĄė×ÓÓė±µĄė×Ó·“Ӧɜ³ÉĮņĖį±µ³Įµķ£®
½ā“š ½ā£ŗ³£ĪĀĻĀ0.01mol•L-1 H2SO4£ØĒæĖį£©ČÜŅŗÖŠc£ØH+£©=0.011mol•L-1£¬ĖµĆ÷µŚŅ»²½µēĄėŹĒĶźČ«µÄ£ŗH2SO4=H++HSO4-£¬µŚ¶ž²½µēĄė²¢²»ĶźČ«£ŗHSO4-?H++SO42-£¬
£Ø1£©ĮņĖįČÜŅŗÖŠµēĄė·½³ĢŹ½ĪŖ£ŗH2SO4=2H++SO42-£¬NaHSO4ČÜŅŗÖŠ£¬ĮņĖįĒāøłĄė×ӵĵēĄė³Ģ¶Č“óÓŚĘäĖ®½ā³Ģ¶Č£¬ČÜŅŗĻŌŹ¾ĖįŠŌ£¬Ōņc£ØH+£©£¾c£ØOH-£©”¢c£ØNa+£©£¾c£ØHSO4-£©£¬ČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”ĪŖ£ŗc£ØNa+£©£¾c£ØHSO4-£©£¾c£ØH+£©£¾c£ØSO42-£©£¾c£ØOH-£©£»
Na2SO4ČÜŅŗÖŠĮņĖįøłĄė×ÓŅ×Ė®½ā£¬ĖłŅŌČÜŅŗÖŠ“ęŌŚĖ®½āĘ½ŗā£ŗSO42-+H2O?OH-+HSO4-£¬µ¼ÖĀČÜŅŗ³ŹČõ¼īŠŌ£¬
¹Ź“š°øĪŖ£ŗH2SO4=2H++SO42-£¬c£ØNa+£©£¾c£ØHSO4-£©£¾c£ØH+£©£¾c£ØSO42-£©£¾c£ØOH-£©£»¼ī£»SO42-+H2O?OH-+HSO4-£»
£Ø2£©0.01mol•L-1 H2SO4£ØĒæĖį£©ČÜŅŗÖŠc£ØH+£©=0.011mol•L-1£¬ĮņĖįµÄµŚŅ»²½µēĄėµÄĒāĄė×ÓÅضČĪŖ0.01mol/L£¬ŌņµŚ¶ž²½µēĄė³öµÄĒāĄė×ÓĪŖ0.001mol/L£¬ĮņĖįµÄµŚŅ»²½µēĄė³öµÄĒāĄė×ÓŅÖÖʵŚ¶ž²½µēĄė£¬ĖłŅŌ0.01mol/LµÄĮņĖįĒāÄĘČÜŅŗÖŠ£¬ĒāĄė×ÓÅضČÓ¦øĆ“óÓŚ0.001mol/L£¬
¹Ź“š°øĪŖ£ŗ£¾£»
£Ø3£©2mol•L-1 NaHSO4ČÜŅŗÓė1mol•L-1 Ba£ØOH£©2ČÜŅŗµČĢå»ż»ģŗĻ£¬·“Ó¦ŗóĮņĖįøłĄė×Ó¹żĮ棬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ2OH-+2HSO4-+Ba2+=BaSO4”ż+2H2O+SO42-£»Čō¼ĢŠųµĪ¼ÓBa£ØOH£©2ČÜŅŗ£¬±µĄė×ÓÓėĮņĖįøłĄė×Ó·“Ӧɜ³ÉĮņĖį±µ³Įµķ£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗSO42-+Ba2+=BaSO4”ż£¬
¹Ź“š°øĪŖ£ŗ2OH-+2HSO4-+Ba2+=BaSO4”ż+2H2O+SO42-£®
µćĘĄ ±¾Ģāæ¼²éĮĖĄė×ÓÅØ¶Č“óŠ”±Č½Ļ”¢ŃĪµÄĖ®½āŌĄķ¼°ĘäÓ¦ÓĆ£¬ĢāÄæÄѶČÖŠµČ£¬Ć÷Č·ĮņĖįµÄµŚ¶ž²½²æ·ÖµēĄėĪŖ½ā“š¹Ų¼ü£¬×¢ŅāÕĘĪÕÅŠ¶ĻĄė×ÓÅØ¶Č“óŠ”³£ÓĆ·½·Ø£¬ŹŌĢā²ąÖŲæ¼²éѧɜµÄ·ÖĪö”¢Ąķ½āÄÜĮ¦£®


 æŚĖćĢāæؼÓÓ¦ÓĆĢā¼ÆѵĻµĮŠ“š°ø
æŚĖćĢāæؼÓÓ¦ÓĆĢā¼ÆѵĻµĮŠ“š°ø ×ŪŗĻ×Ō²āĻµĮŠ“š°ø
×ŪŗĻ×Ō²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

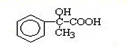
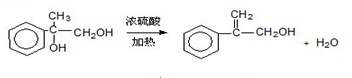
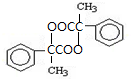
 ³ö·¢ŗĻ³ÉA£¬ĘäŗĻ³ÉĀ·ĻßČēĻĀ£ŗŅŃÖŖ£ŗAŌŚĖįŠŌĢõ¼žĻĀĖ®½āÉś³ÉÓŠ»śĪļBŗĶ¼×“¼£®
³ö·¢ŗĻ³ÉA£¬ĘäŗĻ³ÉĀ·ĻßČēĻĀ£ŗŅŃÖŖ£ŗAŌŚĖįŠŌĢõ¼žĻĀĖ®½āÉś³ÉÓŠ»śĪļBŗĶ¼×“¼£®
 £®
£® £®
£® £®
£®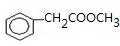 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | HCl”¢HBr”¢HIµÄČŪ”¢·ŠµćŅĄ“ĪÉżøßÓė·Ö×Ó¼ä×÷ÓĆĮ¦“óŠ”ÓŠ¹Ų | |
| B£® | H2OµÄČŪ”¢·ŠµćøßÓŚH2SŹĒÓÉÓŚH2O·Ö×ÓÖ®¼ä“ęŌŚĒā¼ü | |
| C£® | ¼×ĶéæÉÓėĖ®ŠĪ³ÉĒā¼ü | |
| D£® | I2Ņ×ČÜÓŚCCl4æÉŅŌÓĆĻąĖĘĻąČÜŌĄķ½āŹĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| µēĄėÄÜ£ØkJ/mol£© | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |
| B | 738 | 1451 | 7733 | 10540 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaOHŗĶĻ”H2SO4£ŗOH-+H+ØTH2O | |
| B£® | NH4HCO3ČÜŅŗÓė¹żĮæNaOHČÜŅŗ·“Ó¦£ŗNH4++OH-ØTNH3”ü+H2O | |
| C£® | Ģ¼ĖįÄĘČÜŅŗÖŠĶØČėÉŁĮæCO2£ŗCO32-+CO2+H2OØT2HCO3- | |
| D£® | NaHCO3ŗĶH2SO4·“Ó¦£ŗHCO3-+H+ØTH2O+CO2”ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ÓČėÉŁĮæ W£¬Äę·“Ó¦ĖŁĀŹŌö“ó | |
| B£® | ÉżøßĪĀ¶Č£¬Ę½ŗāÄęĻņŅĘ¶Æ | |
| C£® | Ę½ŗāŗó¼ÓČė X£¬ÉĻŹö·“Ó¦µÄ”÷HŌö“ó | |
| D£® | µ±ČŻĘ÷ÖŠĘųĢåŃ¹Ēæ²»±äŹ±£¬·“Ó¦“ļµ½Ę½ŗā |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com