
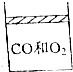
 ��25�棬101KPa�������£�����8L��CO��O2�Ļ������ͨ��һ�����������ƶ������ݻ��ɱ䵫ѹǿ������ܱ������У���ͼ��ʾ�����õ��ȼ��ַ�Ӧ��ָ���ԭ�������������������ܶȱ�Ϊԭ����$\frac{4}{3}$����
��25�棬101KPa�������£�����8L��CO��O2�Ļ������ͨ��һ�����������ƶ������ݻ��ɱ䵫ѹǿ������ܱ������У���ͼ��ʾ�����õ��ȼ��ַ�Ӧ��ָ���ԭ�������������������ܶȱ�Ϊԭ����$\frac{4}{3}$�������� ��1��������Ӧ��2CO+O2$\frac{\underline{\;���\;}}{\;}$2CO2������������������䣬��ַ�Ӧ��ָ���ԭ�������������������ܶȱ�Ϊԭ����$\frac{4}{3}$����Ӧ������Ϊԭ����$\frac{3}{4}$������Ӧ������Ϊ8L��$\frac{3}{4}$=6L�����ò���������μӷ�ӦCO��O2������������ۼ���ԭ���������CO��O2���ܵ�����ȣ�
��2������Ӧ��������ܶ�����ͬ������Ϊ�����ܶȵ�$\frac{29}{3}$������Ӧ���������Է�������Ϊ4��$\frac{29}{3}$����Ӧ������ΪCO2 ��O2��CO2 ��CO���ټ���ƽ����Է�������������֤���
��� �⣺��1������������������䣬��ַ�Ӧ��ָ���ԭ�������������������ܶȱ�Ϊԭ����$\frac{4}{3}$����Ӧ������Ϊԭ����$\frac{3}{4}$������Ӧ������Ϊ8L��$\frac{3}{4}$=6L��
2CO+O2$\frac{\underline{\;���\;}}{\;}$2CO2 �����С
2 1 2 1
4L 2L 4L 8L-6L=2L
��CO��ʣ�࣬��O2Ϊ2L��COΪ8L-2L=6L��CO��O2�������Ϊ6L��2L=3��1��
��O2��ʣ�࣬��COΪ4L��O2Ϊ8L-4L=4L��CO��O2�������Ϊ4L��4L=1��1��
�ʴ�Ϊ��3��1��1��1��
��2������Ӧ��������ܶ�����ͬ������Ϊ�����ܶȵ�$\frac{29}{3}$������Ӧ���������Է�������Ϊ4��$\frac{29}{3}$��
�ɣ�1�������֪������Ӧ��ΪCO2 ��O2������壬�������ʵ���֮��Ϊ4L����4L-2L��=2��1��ƽ����Է�������Ϊ$\frac{44��2+32}{2+1}$=40�������ϣ�
����Ӧ��ΪCO2 ��CO������壬�������ʵ���֮��Ϊ4L����6L-4L��=2��1��ƽ����Է�������Ϊ$\frac{44��2+28}{3}$=4��$\frac{29}{3}$�����ϣ�
�ʷ�Ӧ������ijɷ�ΪCO2 ��CO���������ʵ���֮��Ϊ2��1��
�ʴ�Ϊ��CO2 ��CO��2��1��
���� ���⿼�鰢��٤�����ɼ������ۡ�������йؼ��㣬��Ŀ���漰�����������㣬���ؿ���ѧ�����������������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{20��1{0}^{-3}}{127}$mol | B�� | 20M mol | C�� | $\frac{20��1{0}^{-3}}{M}$mol | D�� | $\frac{20}{M}$mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������˴Ź�������ͼ��ʾ�����ط壬�����֮��Ϊ3��2��3�����л��ﲻ����CH3-O-����A�Ľṹ��ʽΪCH3COOCH2CH3��
������˴Ź�������ͼ��ʾ�����ط壬�����֮��Ϊ3��2��3�����л��ﲻ����CH3-O-����A�Ľṹ��ʽΪCH3COOCH2CH3�� �dz����㾫���㷺����ʳƷ����ױƷ����ҵ���ɴ���Ȼ������ȡ��Ҳ���˹��ϳɣ�ʵ��������ʳƷ������[��Ҫ�ɷ�Ϊ�������ƣ�
�dz����㾫���㷺����ʳƷ����ױƷ����ҵ���ɴ���Ȼ������ȡ��Ҳ���˹��ϳɣ�ʵ��������ʳƷ������[��Ҫ�ɷ�Ϊ�������ƣ�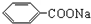 ��]���״�Ϊԭ���Ʊ��������������֪��
��]���״�Ϊԭ���Ʊ��������������֪��| �۵�� | �е�� | ˮ���� | |
| �״� | -97.8 | 64.7 | ���� |
| ������ ��һԪ���ᣩ | 122.4 | 249.3 | ���£�0.17g 100�棺6.8g |
| ��������� | -12.3 | 198 | ���� |

 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | SiO2 | C | Na2O | K2O | Al2O3 | Fe2O3 |
| �������� | 59.20 | 38.80 | 0.25 | 0.50 | 0.64 | 0.16 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
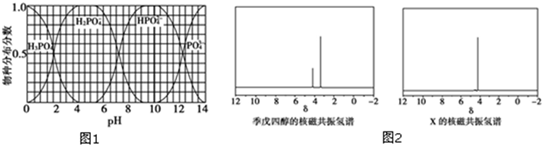
 ���뼾���Ĵ���
���뼾���Ĵ���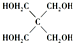 �������ʵ���֮��2��1 ��Ӧ
�������ʵ���֮��2��1 ��Ӧ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����ͭ���仯�������ճ��������������Ź㷺��Ӧ�ã���ش��������⣺
����ͭ���仯�������ճ��������������Ź㷺��Ӧ�ã���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���顡���� | B�� | �����顡������ | ||
| C�� | ��-���ױ�����-���ױ� | D�� | �⡡�ɱ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com