



 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��Ԫ�صĺ�������Ų�ʽ_______________________________________;
��Ԫ�صĺ�������Ų�ʽ_______________________________________;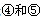 ��һ�����ܵĴ�С��ϵΪ________________(��Ԫ�ط��ű�ʾ)
��һ�����ܵĴ�С��ϵΪ________________(��Ԫ�ط��ű�ʾ)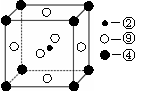
| A�������к������ | B�������ӳɷ�Ӧ |
| C������4���Ҽ���1���м� | D������������ԭ�Ӵ���ͬһ��ƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
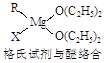
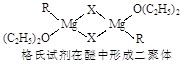
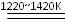 Ti+2MgCl2
Ti+2MgCl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1���ʰ�������д���10�Թ��õ��� |
| B��PCl3����BCl3����������ԭ�ӵ�����㶼�ﵽ8�����ȶ��ṹ |
| C��ά��������ϳ������أ�ͻ�����������л���Ľ��� |
| D���۵��ɸߵ��͵�˳���ǣ����ʯ��̼���裾����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��� | Ԫ�� | �ṹ������ |
| �� | A | A�ڵ��������м����Ӱ뾶��С |
| �� | B | Bԭ���������������ڲ��������1/5 |
| �� | C | C�dz��û��ʵ���ҪԪ��,���ʳ����³���̬ |
| �� | D | ͨ������£�Dû�������ϼۣ�A��B��C������D�γɻ����� |
| �� | E | E�����ڱ��п�������IA�壬Ҳ�������ڢ�A�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com