
 =0.02mol£¬L-¶ą°ĶĻą¶Ō·Ö×ÓÖŹĮæĪŖ
=0.02mol£¬L-¶ą°ĶĻą¶Ō·Ö×ÓÖŹĮæĪŖ =197£¬ÓÖ3.94g L-¶ą°ĶŌŚæÕĘųÖŠĶźČ«Č¼ÉÕæÉÉś³É0.18mol CO2”¢0.448LNH3£Ø±ź×¼×“æö£©”¢1.44g H2O£¬Ōņn£ØNH3£©=
=197£¬ÓÖ3.94g L-¶ą°ĶŌŚæÕĘųÖŠĶźČ«Č¼ÉÕæÉÉś³É0.18mol CO2”¢0.448LNH3£Ø±ź×¼×“æö£©”¢1.44g H2O£¬Ōņn£ØNH3£©= =0.02mol£¬n£ØH£©=2×
=0.02mol£¬n£ØH£©=2×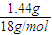 +3×0.02mol=0.22mol£¬
+3×0.02mol=0.22mol£¬ =0.08mol£¬ĖłŅŌ£¬1 mol L-¶ą°Ķŗ¬ÓŠn£ØC£©=9 mol£¬n£ØH£©=11 mol£¬n£ØN£©=l mol£¬n£ØO£©=4 mol£¬ĖłŅŌL-¶ą°ĶµÄ·Ö×ÓŹ½ĪŖC9H11O4N£»
=0.08mol£¬ĖłŅŌ£¬1 mol L-¶ą°Ķŗ¬ÓŠn£ØC£©=9 mol£¬n£ØH£©=11 mol£¬n£ØN£©=l mol£¬n£ØO£©=4 mol£¬ĖłŅŌL-¶ą°ĶµÄ·Ö×ÓŹ½ĪŖC9H11O4N£» =0.02mol£¬L-¶ą°ĶĻą¶Ō·Ö×ÓÖŹĮæĪŖ
=0.02mol£¬L-¶ą°ĶĻą¶Ō·Ö×ÓÖŹĮæĪŖ =197£¬ÓÖ3.94g L-¶ą°ĶŌŚæÕĘųÖŠĶźČ«Č¼ÉÕæÉÉś³É0.18mol CO2”¢0.448LNH3£Ø±ź×¼×“æö£©”¢1.44g H2O£¬Ōņn£ØNH3£©=
=197£¬ÓÖ3.94g L-¶ą°ĶŌŚæÕĘųÖŠĶźČ«Č¼ÉÕæÉÉś³É0.18mol CO2”¢0.448LNH3£Ø±ź×¼×“æö£©”¢1.44g H2O£¬Ōņn£ØNH3£©= =0.02mol£¬n£ØH£©=2×
=0.02mol£¬n£ØH£©=2× +3×0.02mol=0.22mol£¬
+3×0.02mol=0.22mol£¬ =0.08mol£¬
=0.08mol£¬ £¬
£¬ £»
£»

 ½Ģ²ÄČ«½ā×Ö“Ź¾äĘŖĻµĮŠ“š°ø
½Ģ²ÄČ«½ā×Ö“Ź¾äĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ÕāÖÖŅ©ĪļµÄŃŠÖĘŹĒ»łÓŚ»ńµĆ2000ÄźÅµ±“¶ūÉśĄķѧ»ņŅ½Ń§½±ŗĶ»ńµĆ2001ÄźÅµ±“¶ū»Æѧ½±µÄŃŠ¾æ³É¹ū£®ĻĀĮŠ¹ŲÓŚL-¶ą°ĶĖį¼īŠŌµÄŠšŹöÕżČ·µÄŹĒ£Ø””””£©
ÕāÖÖŅ©ĪļµÄŃŠÖĘŹĒ»łÓŚ»ńµĆ2000ÄźÅµ±“¶ūÉśĄķѧ»ņŅ½Ń§½±ŗĶ»ńµĆ2001ÄźÅµ±“¶ū»Æѧ½±µÄŃŠ¾æ³É¹ū£®ĻĀĮŠ¹ŲÓŚL-¶ą°ĶĖį¼īŠŌµÄŠšŹöÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
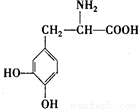
 L-¶ą°ĶŹĒŅ»ÖÖÓŠ»śĪļ£¬ĖüæÉÓĆÓŚÅĮ½šÉ×ŪŗĻÖ¢µÄÖĪĮĘ£¬Ęä½į¹¹¼ņŹ½ČēĶ¼£ŗÕāÖÖŅ©ĪļµÄŃŠÖĘŹĒ»łÓŚ»ńµĆ2000ÄźÅµ±“¶ūÉśĄķѧ»ņŅ½Ń§½±ŗĶ»ńµĆ2001ÄźÅµ±“¶ū»Æѧ½±µÄŃŠ¾æ³É¹ū£®ĻĀĮŠ¹ŲÓŚL-¶ą°ĶµÄŠšŹö²»ÕżČ·µÄŹĒ£Ø””””£©
L-¶ą°ĶŹĒŅ»ÖÖÓŠ»śĪļ£¬ĖüæÉÓĆÓŚÅĮ½šÉ×ŪŗĻÖ¢µÄÖĪĮĘ£¬Ęä½į¹¹¼ņŹ½ČēĶ¼£ŗÕāÖÖŅ©ĪļµÄŃŠÖĘŹĒ»łÓŚ»ńµĆ2000ÄźÅµ±“¶ūÉśĄķѧ»ņŅ½Ń§½±ŗĶ»ńµĆ2001ÄźÅµ±“¶ū»Æѧ½±µÄŃŠ¾æ³É¹ū£®ĻĀĮŠ¹ŲÓŚL-¶ą°ĶµÄŠšŹö²»ÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
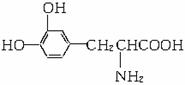
ĻĀĮŠ¹ŲÓŚL¶ą°ĶŠŌÖŹµÄĶĘ²āÕżČ·µÄŹĒ£Ø £©
A.²»æÉÄÜŠĪ³Éøß·Ö×Ó»ÆŗĻĪļ B.²»Ņ×±»Ńõ»Æ
C.Ö»ÓŠĖįŠŌ D.ÄÜÓėäåĖ®·¢ÉśČ”“ś·“Ó¦
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com