
| ʵ����� | ����Һ�����mL�� | �����������Һ�������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.50 | 20.55 |
| 2 | 20.00 | 8.00 | 26.00 |
| 3 | 20.00 | 1.40 | 21.35 |
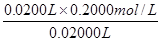 ��0.2000mol/L��
��0.2000mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaOH��Һ |
| B��NaHSO4��Һ |
| C��NH4Cl��Һ |
| D��0.001mol��L-1��CH3COOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ij���ʵ���ҺpH <7���������һ�������ǿ�������� |
| B��AgCl����ͬ���ʵ���Ũ�ȵ�CaCl2��NaCl��Һ�е��ܽ����ͬ |
| C��pH=5.6��CH3COOH��CH3COONa�����Һ�У�c(Na+)> c(CH3COO��) |
| D��pH=4.5�ķ���֭��c(H+)��pH=6.5��ţ����c(H+)��100�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��pH��7 | B��c(H+)��c(OH-) | C��pH��ֽ����ɫ | D��ʯ����Һ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c (CH3COO��)="c" (Na+) | B��c (OH��)��c (H+) |
| C��c (CH3COOH)��c (CH3COO��) | D��c (CH3COOH)+c (CH3COO��)=0.01mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��a��7ʱ��ˮ�ĵ����ܵ����� | B��a��7ʱ��ˮ�ĵ����ܵ����� |
| C��a��7ʱ����Һ��pH����Ϊa | D��a��7ʱ����Һ��pH����Ϊ14��a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(H+)=10-7mol/L����Һ | B��pH=7����Һ |
| C��c(H+)/c(OH-)=10-14����Һ | D����ˮ���Ȼ�淋Ļ��Һ��c(NH4+)=c(Cl-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� | �ζ����ʱ������NaOH��Һ�������mL�� | ����������Һ�������mL�� |
| 1 | 0.10 | 20.02 | 20.00 |
| 2 | 0.10 | 20.00 | 20.00 |
| 3 | 0.10 | 19.00 | 20.00 |
| 4 | 0.10 | 19.98 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
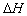 �ľ���ֵ����ȷ���� ��
�ľ���ֵ����ȷ���� ��A��C2H5OH(l)��3O2(g) �� 2CO2 (g)��3H2O(g) kJ/mol��ȼ���ȣ� kJ/mol��ȼ���ȣ� |
B��NaOH(aq)��HCl (aq) �� NaCl (aq) ��H2O(l)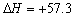 kJ/mol���к��ȣ� kJ/mol���к��ȣ� |
C��S(s)��O2(g) �� SO2(g)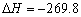 kJ/mol����Ӧ�ȣ� kJ/mol����Ӧ�ȣ� |
D��Fe��S �� FeS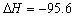 kJ/mol����Ӧ�ȣ� kJ/mol����Ӧ�ȣ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com