
·ÖĪö £Ø1£©Ļ”µÄĖį¼ī·“Ӧɜ³É1molŹ±µÄČČĮæ±ä»ÆĪŖÖŠŗĶČČ£¬1mol H2SO4ÓėNaOHČÜŅŗĒ”ŗĆĶźČ«·“Ó¦Ź±£¬·Å³ö114.6kJČČĮ棬ĒŅÉś³É2molĖ®£»
£Ø2£©¢ŁCH3COOH£Øl£©+2O2£Øg£©ØT2CO2£Øg£©+2H2O£Øl£©”÷H1=-870.3kJ/mol
¢ŚC£Øs£©+O2£Øg£©ØTCO2£Øg£©”÷H2=-393.5kJ/mol
¢ŪH2£Øg£©+$\frac{1}{2}$O2£Øg£©ØTH2O£Øl £©”÷H3=-285.8kJ/mol
ÓÉøĒĖ¹¶ØĀÉæÉÖŖ£¬¢Ś”Į2+¢Ū”Į2-¢ŁµĆµ½2C£Øs£©+2H2£Øg£©+O2£Øg£©ØTCH3COOH£Øl£©£»
£Ø3£©¢ŁC£Øs£¬ŹÆÄ«£©+O2£Øg£©=CO2£Øg£©”÷H1=Q1kJ•mol-1£¬
¢Ś2CO2£Øg£©+H2 £Øg£©=C2H2£Øg£©+2O2£Øg£©”÷H2=Q2kJ•mol-1£®
ÓÉøĒĖ¹¶ØĀÉæÉÖŖ¢Ł”Į2+¢ŚµĆµ½2C£ØŹÆÄ«£¬s£©+H2£Øg£©ØTC2H2£Øg£©£¬ŅŌ“ĖĄ“½ā“š£®
½ā“š ½ā£ŗ£Ø1£©Ļ”µÄĖį¼ī·“Ӧɜ³É1molŹ±µÄČČĮæ±ä»ÆĪŖÖŠŗĶČČ£¬1mol H2SO4ÓėNaOHČÜŅŗĒ”ŗĆĶźČ«·“Ó¦Ź±£¬·Å³ö114.6kJČČĮ棬ĒŅÉś³É2molĖ®£¬ŌņÖŠŗĶČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ$\frac{1}{2}$H2SO4£Øaq£©+NaOH£Øaq£©ØT$\frac{1}{2}$Na2SO4£Øaq£©+H2O£Øl£©”÷H=-57.3 kJ/mol£¬
¹Ź“š°øĪŖ£ŗ$\frac{1}{2}$H2SO4£Øaq£©+NaOH£Øaq£©ØT$\frac{1}{2}$Na2SO4£Øaq£©+H2O£Øl£©”÷H=-57.3 kJ/mol£»
£Ø2£©¢ŁCH3COOH£Øl£©+2O2£Øg£©ØT2CO2£Øg£©+2H2O£Øl£©”÷H1=-870.3kJ/mol
¢ŚC£Øs£©+O2£Øg£©ØTCO2£Øg£©”÷H2=-393.5kJ/mol
¢ŪH2£Øg£©+$\frac{1}{2}$O2£Øg£©ØTH2O£Øl £©”÷H3=-285.8kJ/mol
ÓÉøĒĖ¹¶ØĀÉæÉÖŖ£¬¢Ś”Į2+¢Ū”Į2-¢ŁµĆµ½2C£Øs£©+2H2£Øg£©+O2£Øg£©ØTCH3COOH£Øl£©”÷H=£Ø-870.3kJ/mol£©”Į2+£Ø-393.5kJ/mol£©”Į2-£Ø-285.8kJ/mol£©=-488.3 kJ/mol£¬
¹Ź“š°øĪŖ£ŗ2C£Øs£©+2H2£Øg£©+O2£Øg£©ØTCH3COOH£Øl£©”÷H=-488.3 kJ/mol£»
£Ø3£©¢ŁC£Øs£¬ŹÆÄ«£©+O2£Øg£©=CO2£Øg£©”÷H1=Q1kJ•mol-1£¬
¢Ś2CO2£Øg£©+H2 £Øg£©=C2H2£Øg£©+2O2£Øg£©”÷H2=Q2kJ•mol-1£®
ÓÉøĒĖ¹¶ØĀÉæÉÖŖ¢Ł”Į2+¢ŚµĆµ½2C£ØŹÆÄ«£¬s£©+H2£Øg£©ØTC2H2£Øg£©”÷H=£Ø2Q1+Q2£© kJ/mol£¬
¹Ź“š°øĪŖ£ŗ2C£ØŹÆÄ«£¬s£©+H2£Øg£©ØTC2H2£Øg£©”÷H=£Ø2Q1+Q2£© kJ/mol£®
µćĘĄ ±¾Ģā漲鷓ӦČČÓėģŹ±ä£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕČČ»Æѧ·½³ĢŹ½µÄŹéŠ“”¢·“Ó¦ÖŠÄÜĮæ±ä»ÆĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪöÓėÓ¦ÓĆÄÜĮ¦µÄ漲飬עŅāøĒĖ¹¶ØĀÉÓ¦ÓĆ£¬ĢāÄæÄŃ¶Č²»“ó£®


 ѧĮ·æģ³µµĄæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ѧĮ·æģ³µµĄæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ČÜŅŗÖŠŗ¬ÓŠ2mol K2SO4 | B£® | æɽ«2mol K2SO4ČÜÓŚ1LĖ®ÖŠÖĘµĆ | ||
| C£® | ČÜŅŗÖŠc£ØK+ £©=4 mol•L-1 | D£® | 1L ČÜŅŗÖŠŗ¬4molK+£¬4molSO42- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā

| ¼Ł Éč | ²Ł ×÷ | ĻÖ Ļó | Ō Ąķ |
| Na2S2O4ĪŖĒæ¼īČõĖįŃĪ£¬ĘäČÜŅŗĪŖ¼īŠŌ£® | ȔɣĮæČÜŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼Ó ¢Ś×ĻÉ«ŹÆČļŹŌŅŗ | ČÜŅŗ±ä³ÉĄ¶É« | S2O42-Ė®½ā£¬Ź¹ČÜŅŗ³É¼īŠŌ |
| ¢ŁNa2S2O4¾ßÓŠ»¹ŌŠŌ | ȔɣĮæČÜŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼Ó¹żĮæŠĀÖĘĀČĖ®£¬ŌŁµĪ¼Ó BaCl2 ČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É | øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŅĄ“ĪĪŖ£ŗ¢Ū4H2O+S2O42-+3Cl2=2SO42-+6Cl-+8H+£¬¢ÜBa2++SO42-=BaSO4”ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
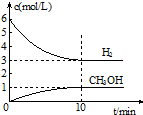 T”ꏱ£¬ŌŚ1LµÄĆܱÕČŻĘ÷ÖŠ³äČė2mol CO2ŗĶ6mol H2£¬·¢Éś·“Ó¦£ŗCO2£Øg£©+3H2£Øg£©?CH3OH£Øg£©+H2O£Øg£©”÷H=-49.0kJ•mol-1£®²āµĆH2ŗĶCH3OH£Øg£©µÄÅضČĖꏱ¼ä±ä»ÆĒéæöČēĶ¼ĖłŹ¾£ŗĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©
T”ꏱ£¬ŌŚ1LµÄĆܱÕČŻĘ÷ÖŠ³äČė2mol CO2ŗĶ6mol H2£¬·¢Éś·“Ó¦£ŗCO2£Øg£©+3H2£Øg£©?CH3OH£Øg£©+H2O£Øg£©”÷H=-49.0kJ•mol-1£®²āµĆH2ŗĶCH3OH£Øg£©µÄÅضČĖꏱ¼ä±ä»ÆĒéæöČēĶ¼ĖłŹ¾£ŗĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | 0”«10minÄŚv£ØH2£©=0.3 mol•L-1•min-1 | |
| B£® | T”ꏱ£¬Ę½ŗā³£ŹżK=$\frac{1}{27}$£¬CO2ÓėH2µÄ×Ŗ»ÆĀŹĻąµČ | |
| C£® | T”ꏱ£¬µ±ÓŠ32 g CH3OHÉś³ÉŹ±£¬·Å³ö49.0 kJµÄČČĮæ | |
| D£® | “ļµ½Ę½ŗāŗó£¬ÉżøßĪĀ¶Č»ņŌŁ³äČėCO2ĘųĢ壬¶¼æÉŅŌĢįøßH2µÄ×Ŗ»ÆĀŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| µĪ¶Ø“ĪŹż | “ż²ā°±Ė®ČÜŅŗµÄĢå»ż/mL | 0.10mol/LŃĪĖįµÄĢå»ż/mL | ||
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ČÜŅŗĢå»ż/mL | ||
| µŚŅ»“Ī | 25.00 | 0.00 | 26.11 | 26.11 |
| µŚ¶ž“Ī | 25.00 | 1.56 | 30.30 | 28.74 |
| µŚČż“Ī | 25.00 | 0.22 | 26.31 | 26.09 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
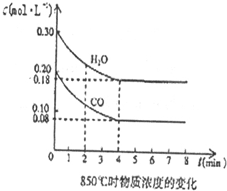
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com