
��14�֣�����ʽ��3�֣�����ÿ��2�֣�2-�ǻ������������ƻ���е�һ���л�����ṹ��ʽ�ǣ� ��
��
��1��2-�ǻ��������к��еĹ����������� ����һ�����������ɷ�����ѧ��Ӧ�������� ������ţ���
| A��ˮ�ⷴӦ | B��ȡ����Ӧ | C���ӳɷ�Ӧ | D����ȥ��Ӧ E.�Ӿ۷�Ӧ F.�кͷ�Ӧ |
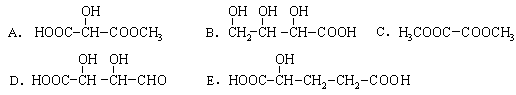
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�����ʽ��3�֣�����ÿ��2�֣�2-�ǻ������������ƻ���е�һ���л�����ṹ��ʽ�ǣ�
��
��1��2-�ǻ��������к��еĹ����������� ����һ�����������ɷ�����ѧ��Ӧ�������� ������ţ���
A.ˮ�ⷴӦ B.ȡ����Ӧ C.�ӳɷ�Ӧ D.��ȥ��Ӧ E.�Ӿ۷�Ӧ F.�кͷ�Ӧ
��2������������2-�ǻ������ụΪͬ���칹����� ������ţ���
��3��д��2-�ǻ����������Ҵ���һ�������·�����Ӧ�Ļ�ѧ����ʽ��
��
��4��2-�ǻ���������һ�������¿��Ƶ��л���X��X��ʹ������Ȼ�̼��Һ��ɫ��
д��X�Ľṹ��ʽ �������� ���仯ѧ����ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȹ㶫����һ��ʵ��ѧУ�߶���ѧ��3���¿���ѧ�Ծ��������棩 ���ͣ������
��14�֣�����ʽ��3�֣�����ÿ��2�֣�2-�ǻ������������ƻ���е�һ���л�����ṹ��ʽ�ǣ�
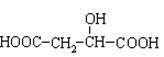 ��
��
��1��2-�ǻ��������к��еĹ����������� ����һ�����������ɷ�����ѧ��Ӧ�������� ������ţ���
A.ˮ�ⷴӦ B.ȡ����Ӧ C.�ӳɷ�Ӧ D.��ȥ��Ӧ E.�Ӿ۷�Ӧ F.�кͷ�Ӧ
��2������������2-�ǻ������ụΪͬ���칹����� ������ţ���
��3��д��2-�ǻ����������Ҵ���һ�������·�����Ӧ�Ļ�ѧ����ʽ��
��
��4��2-�ǻ���������һ�������¿��Ƶ��л���X��X��ʹ������Ȼ�̼��Һ��ɫ��
д��X�Ľṹ��ʽ �������� ���仯ѧ����ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ֣���и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(14��)��һ����Һ����֪���п��ܺ���Fe3����Mg2����Cu2����Al3����NH4��������һ�ֵ���ɫ��ĩ����ʱ�������д̼�����ζ�Ļ������ų���ͬʱ���ɰ�ɫ������������0.4 mol����ɫ��ĩʱ����������0.3 mol���������뵭��ɫ��ĩʱ�������̼�����ζ�����壬�Ҽ��뵭��ɫ��ĩʱ������ɫ������������ͼ��ʾ��
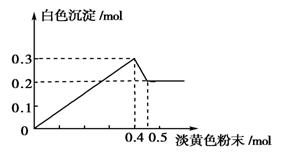
��������ش��������⣺
(1)����ɫ��ĩΪ____________(������)��
(2)��Һ�п϶���______________���ӣ��϶�û��__________���ӡ�
(3)��Һ�и����ӵ����ʵ���֮��Ϊ
________________________________________________________________________��
(4)д�����з�Ӧ����ʽ��
�ٵ���ɫ��ĩ��ˮ��Ӧ�Ļ�ѧ����ʽ��____________________________________��
�ڴ̼�����ζ��������������ӷ���ʽ��______________________________________��
�۳������ּ���ʱ�����ӷ���ʽ��_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꼪��ʡ������һ��ģ�⻯ѧ�Ծ� ���ͣ������
(14��)(��)����4�֣�ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ�������ʣ�O2��H2CrO4��Cr(OH)3��H2O��H2O2
��֪�÷�Ӧ��H2O2ֻ�������¹��̣�H2O2�� O2
��1��д���÷�Ӧ�Ļ�ѧ����ʽ________________________��
��2���÷�Ӧ�еĻ�ԭ���� ����ԭ������____________��
(��)��10�֣���ֻ�Լ�ƿ�зֱ�ʢװ��NaNO3��Һ��Na2CO3��Һ��Na2SO4��Һ��NaCl��Һ������μ�����������Һ�ֱ������и��⡣
����֧�Թ��зֱ�ȡ������Һ��1mL��������ʵ�顣
��1������֧�Թ��зֱ���� ������ ����ģ��� ��
��2����ʣ����֧�Թ��зֱ���� ������ ����ģ��� ��
��3����ʣ����֧�Թ��зֱ���� ������ ����ģ��� ������ʵ���ж�û������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com