
����ˮAlCl3����ķе�Ϊ![]() Al3++3Cl-
Al3++3Cl-
��PbSO4������ˮ�������ڴ�������Һ����Ӧ�Ļ�ѧ����ʽΪ��PbSO4+2CH3COONa![]() Na2SO4+(CH3COO)2Pb
Na2SO4+(CH3COO)2Pb
���й���Al(OH)3��AlCl3��(CH3COO)2Pb��˵����ȷ����( )
A.��Ϊǿ����� B.��Ϊ�������
C.��Ϊ���ӻ����� D.��Ϊ���ۻ�����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PbSO4+2CH3COONa====Na2SO4+(CH3COO)2Pb���й���Al(OH)3��AlCl3�ͣ�CH3COO��2Pb��˵����ȷ���ǣ� ��
A.��Ϊǿ����� B.��Ϊ�������
C.��Ϊ���ӻ����� D.��Ϊ���ۻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ���ض��и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ���ѡ��
��֪Ba[Al(OH)4]2������ˮ����ͼ��ʾ������A12(SO4)3��Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ�������й�������ȷ����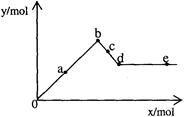
A a��bʱ���������ʵ�����A1(OH)3��BaSO4��
B d��eʱ��Һ�����ӵ����ʵ�����Ba2�����ܵ���OH��
C a��dʱ���������ʵ�����BaSO4����С��A1(OH)3
D c��dʱ��Һ�����ӵ����ʵ�����[Al(OH)4]����Ba2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�츣��ʡ�ĵ���У������ҵ�ǰģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��15�֣��ҹ���������Ҫ�ɷ�ΪFeTiO3��������Al2O3��SiO2�ȣ��Ĵ�����������λ������������ȡTiO2����Ʒ������������ĵ��������£�
��֪��Al(OH)3��Ksp= 1.3��10-33��Fe(OH)2��Ksp= 1.6��10-14��
��1����ҺI��Ҫ����TiO2+��SO42����Fe2+�� �������ӷ��ţ���
��2������PHӦ���ʹ�� ��
| A��Fe | B��Ca(OH)2 | C��NH3?H2O | D��NaOH |
 ���ķ�ӦΪ��
���ķ�ӦΪ�� 92�������Ʒ����������茶��崿�ȵļ���ʽ�ɱ���Ϊ��w%= ��
92�������Ʒ����������茶��崿�ȵļ���ʽ�ɱ���Ϊ��w%= ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ӱ�ʡ������ѧ�ڵ��Ĵ��¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
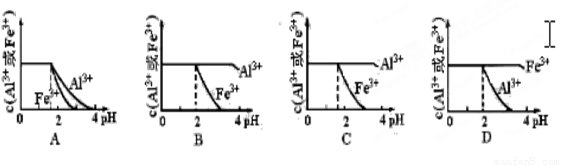
��֪Ksp[Al(OH)3]=1.3��10-33 mol4��L-4�� Ksp[Fe(OH)3]=4.0��10-38 mol4��L-4������pH=0Ũ�Ⱦ�Ϊ0.04mol��L-1��Al3+��Fe3+��Һ�м���NaOH��Һ���Ե���pH(����Һ�������)���ù�����Al3+��Fe3+��Ũ����pH��ϵ��ȷ���ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ2010-2011ѧ�������һ��������� ���ͣ������
�Ķ��±��ж���������Ԫ�ص������Ϣ��
|
Ԫ�ش��� |
�����Ϣ |
|
A |
A�ĵ���������ˮ���ҷ�Ӧ���õ�ǿ������Һ |
|
B |
B��ԭ�����������������ڲ������������ |
|
C |
�ڵ�3����Ԫ���У�C�ļ����Ӱ뾶��С |
|
D |
A��B��D��ɵ�36���ӵĻ�����X�Ǽ�������������Ҫ�ɷ� |
|
E |
�����л����ж�����EԪ�� |
��ش�(1)����һ�������£�B2��C�ĵ����ں�ˮ�п��γ�ԭ��أ�Ϊ���ͺ�ˮ������ṩ��Դ��д����ԭ��������ĵ缫��Ӧʽ ��
(2)���ö��Ե缫��⻯����AD��ˮ��Һ���÷�Ӧ�Ļ�ѧ����ʽΪ��
��������������
(3)�������£�0��1 mol��L-1X��Һ��pH 7(�>������=����<��)��ԭ���� (�����ӷ���ʽ˵��)��
(4)����֪Ksp[Al(OH)3]=1.3��10��33��Ksp[Fe(OH)3]=4.0��10��38��
����pH=0��Ũ�Ⱦ�Ϊ0��04mol��L-1��Al3+��Fe3+��Һ�м���A������������Ӧˮ�������Һ���Ե���pH(����Һ�������)���ù�����Al3+��Fe3+��Ũ����pH��ϵ��ȷ����
(����ĸ����)��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com