
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�EԪ���ǵ�������Ԫ����δ�ɶԵ���������Ԫ�أ�ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1��1mol�������������AgNO3��Һ��Ӧ����������3molAgCl��Fԭ�ӵ�һ�ֺ��ص�������Ϊ65��������Ϊ 36�����������������ش��������⣺������ʱҪ��Ԫ�ط��ű�ʾ��
��1��B�⻯����HCl��Ӧ���ɵĺ���BԪ�����ӵĿռ乹���� .FԪ��ԭ�ӵ�����������Ϊ ����
��2��B3-���ӷֱ���AC2����B��C��ɵ���̬�����ﻥΪ�ȵ����壬��B��C��ɵĻ����ﻯѧʽΪ ��B3-���ӻ����Ժ�һ�������ӻ�Ϊ�ȵ����壬�������ӵ���ʽΪ �����������ӳ����ڼ����ճ������е�һ�ֽ��������ӣ�����������ӷ���Ϊ
��3��A��B��C�ĵ�һ��������С�����˳��Ϊ
��4��E3+�ĺ�������Ų�ʽ�� ��ECl3�γɵ�����λ������ﻯѧʽΪ ��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
��6����F��+1��������ľ����ṹ��ͼ��FΪ���������ڡ����ס���
(15�֣���1������������(1��) 1 (1��) ��2��N2O(1��) 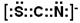 (1��) Fe3+ (1��)
(1��) Fe3+ (1��)
��3��C��O��N (2��) ��4��1s22s22p63s23p63d3 (2��) [Cr(NH3)4(H2O)2]Cl3 (2��)
��5��4Mg��10HNO3��4Mg(NO3)2��NH4NO3��3H2O (2��) ��6���� ( 2��)
�������������A��B��C��ͬһ���ڵķǽ���Ԫ��, AC2Ϊ�Ǽ��Է��ӣ����A��̼Ԫ�أ�C����Ԫ�ء�B��ԭ����������A��C���ۣ�����B�ǵ�Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ������D��þԪ�ء�EԪ���ǵ�������Ԫ����δ�ɶԵ���������Ԫ�أ���E�Ǹ�Ԫ�ء�Fԭ�ӵ�һ�ֺ��ص�������Ϊ65��������Ϊ 36����F��ԭ��������65��36��29����F��ͭԪ�ء�
��1��B�⻯���ǰ�������HCl��Ӧ���ɵĺ���BԪ��������NH4������ռ乹�������������ͣ����ݹ���ԭ����֪��ͭԭ�ӵ�����������4s1������������������1����
��2��ԭ�����ͼ۵������ֱ���ȵ��ǵȵ����壬B3-���Ӻ���3��5��1��16���۵��ӣ�����B3-���ӷֱ���AC2����B��C��ɵ���̬�����ﻥΪ�ȵ����壬��B��C��ɵĻ����ﻯѧʽΪN2O��B3-���ӻ����Ժ�һ�������ӻ�Ϊ�ȵ����壬����������Ӧ����SCN�����ӣ������ʽΪ ���������ӳ����ڼ���Fe3����
���������ӳ����ڼ���Fe3����
��3��ͬ���ڵ�һ������������Ҿ����������ƣ����Ե�һ������O��C�����ڵ�Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܴ�������Ԫ�أ�����A��B��C�ĵ�һ��������С�����˳��ΪC��O��N��
��4�����ݹ���ԭ����֪��E3+�ĺ�������Ų�ʽ��1s22s22p63s23p63d3��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1��1mol�������������AgNO3��Һ��Ӧ����������3molAgCl����˵�������Ӳ�������λ�����γɡ�����CrԪ�صĻ��ϼ��ǣ�3�ۣ��������Ը���λ�������к���6�����壬���а�����4����ˮ��2������˸���λ������Ļ�ѧʽ��[Cr(NH3)4(H2O)2]Cl3��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ����ڵ�Ԫ�ص���ͼ��ǣ�3�ۣ���˵����ԭ����������泥����Ը÷�Ӧ�Ļ�ѧ����ʽ��4Mg��10HNO3��4Mg(NO3)2��NH4NO3��3H2O��
��6�����ݾ����Ľṹ����Ͼ�̯����֪�����������8��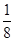 ��1��2�������������4���������ߵĸ���֮����1:2������ΪF��+1����������Cu2O������F�Ǻ���
��1��2�������������4���������ߵĸ���֮����1:2������ΪF��+1����������Cu2O������F�Ǻ���
���㣺����Ԫ�����ڱ��Ľṹ��Ԫ�������ɡ���������Ų�����һ�����ܡ��ȵ����塢����ʽ���ռ乹�͡�����ṹ�Լ�������ԭ��Ӧ����ʽ����д��


 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
ͼ�и���������ѧ��ѧ�г��������ʣ��ס��Ҿ������ӻ���������������Ӹ�����Ϊ1��1�����Ƿ��ͷ۵���Ҫ�ɷ֣�����һ�ֳ��õĻ��ʣ�B��D���³�ѹ�������塣��ش��������⣺
��1����������________��
��2��A��D���ʵ�ˮ��Һ�ֱ�����̪��Һ����Һ���Ժ�ɫ����ԭ��________(���ͬ������ͬ��)��
��3�����ڳ�ʪ�Ŀ����лỺ���ֽ⣬A�����տ����е�ˮ�֣�A��nH2O===A��nH2O(nΪƽ��ֵ��n��10)��ȡû�����Ʊ��ܵļ���Ʒ9.16 g������ˮ�Ƴ���Һ����������ϡ���Ტ��ͣ�ؽ��裬�����������������ɵ�B�����(��״��)���±���(����ˮ�е�B����)
| ��������(mL) | 4 | 8 | 15 | 20 | 50 | 120 | 150 |
| ����B�����(mL) | 0 | 0 | 112 | 224 | 896 | 2240 | 2240 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣺
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�ء� |
| BԪ��ԭ�ӵĺ���p��������s��������1�� |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ�I1=738kJ/mol I2=1451J/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ��������� |
| EԪ�ص������������������IJ�Ϊ4�� |
| F��ǰ�������е縺����С��Ԫ�ء� |
| G�����ڱ��ĵ����С� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����Ԫ��X��Y��Z��Wλ��Ԫ�����ڱ���ǰ�����ڣ���֪���ǵĺ˵�����������ӣ��Һ˵����֮��Ϊ51��Yԭ�ӵ�L��p�������2�����ӣ�Zԭ����Yԭ�ӵļ۲��������ͬ��Wԭ�ӵ�L�������������������֮��Ϊ4��1����d����еĵ�����������������֮��Ϊ5��1��
��1��Y��Z�ɷֱ���X�γ�ֻ��һ������ԭ�ӵĹ��ۻ�����a��b�����ǵķ���ʽ�ֱ���_____��_______���ӻ�����ֱ���________��_________��a���ӵ�����ṹ��____________��
��2��Y������������Z�����������ľ������ͷֱ���_______���塢_______���壻
��3��X����������Y���⻯���У����Ӽ��Խ�С����(�����ʽ) ��
��4��Y��Z�Ƚϣ��縺�Խϴ����____________��
��5��W��Ԫ�ط����� ����+2�����ӵĺ�������Ų�ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
������Ԫ��A��B��C��D��Eԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Bԭ�ӵļ۵��������ڸ�Ԫ����ͻ��ϼ۵ľ���ֵ��C��D���γ�D2C��D2C2���ֻ������D��ͬ�����н�������ǿ��Ԫ�أ�E�ĸ�һ��������C��A�γɵ�ij�ֻ�������Ӻ�����ͬ�ĵ�������
��1��A��C��D�γɵĻ������к��еĻ�ѧ������Ϊ ��
��2����֪����E��E��2E������H����a kJ��mol-1
�� 2A����A��A����H=��b kJ��mol-1
��E����A����A��E����H=��c kJ��mol-1����������ʾ�γɹ��ۼ����ṩ�ĵ��ӣ�
д��298Kʱ��A2��E2��Ӧ���Ȼ�ѧ����ʽ ��
��3����ij�¶��¡��ݻ���Ϊ2L�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��º��ݣ�ʹ֮������Ӧ��2A2��g����BC��g�� X��g������H����dJ��mol��1��d��0��XΪA��B��C����Ԫ����ɵ�һ�ֻ��������ʼͶ����������ﵽƽ��ʱ���й��������£�
X��g������H����dJ��mol��1��d��0��XΪA��B��C����Ԫ����ɵ�һ�ֻ��������ʼͶ����������ﵽƽ��ʱ���й��������£�
| ʵ�� | �� | �� | �� |
| ��ʼͶ�� | 2 molA2��1 molBC | 1 molX | 4 molA2��2 molBC |
| ƽ��ʱn��X�� | 0.5mol | n2 | n3 |
| ��Ӧ�������仯 | �ų�Q1kJ | ����Q2kJ | �ų�Q3kJ |
| ��ϵ��ѹǿ | P1 | P2 | P3 |
| ��Ӧ���ת���� | ��1 | ��2 | ��3 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����ѧ����ѡ��5���л���ѧ������(15��)
������H��������·�ߺϳɣ�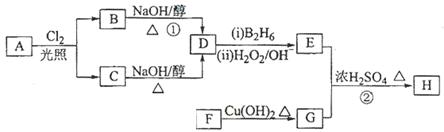
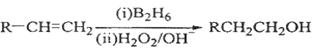
��֪��
��ش��������⣺
��1����״����11.2 L��A�������г��ȼ�տ�������88 g CO2��45 g H2O����A���ӽṹ����3��������A�Ľṹ��ʽΪ ��
��2��B��C��Ϊһ�ȴ�����D������(ϵͳ����)Ϊ ��
��3���ڴ���������1 mol F��2 mol H2��Ӧ������3��������1��������F�Ľṹ��ʽ�� ��
��4����Ӧ�ٵķ�Ӧ������ ��
��5����Ӧ�ڵĻ�ѧ����ʽΪ ��
��6��д��������G������ͬ�����ŵķ�����ͬ���칹��Ľṹ��ʽ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����ѧ����ѡ��5���л���ѧ��������15�֣�
������H��������·�ߺϳɣ�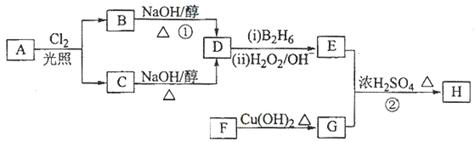
��֪��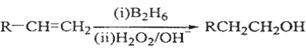
�ش��������⣺
��1��11.2L����״��������A�������г��ȼ�տ�������88gCO2��45gH2O����A���ӽṹ����3��������A�Ľṹ��ʽΪ ��
��2��B��C��Ϊһ�ȴ�����D�����ƣ�ϵͳ������Ϊ ��
��3���ڴ���������1molF��2molH2��Ӧ������3��������1��������F�Ľṹ��ʽ
�� ��
��4����Ӧ�ٵķ�Ӧ������ ��
��5����Ӧ�ڵĻ�ѧ����ʽΪ ��
��6��д��������G������ͬ�����ŵķ�����ͬ���칹��Ľṹ��ʽ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
���ɽ����ٴ���ż����Ӧ�ǽ������л��ϳɵ��ȵ�֮һ���練Ӧ�٣�
������II�������ºϳ�·��ã�
��1��������I���������ŵ�����Ϊ ��������II�ķ���ʽΪ ��
��2��������IV�Ľṹ��ʽΪ ��ijͬѧ�������辭��Ӧ�ڡ��ۡ��ܺ͢ݣ�ֱ��������KMnO4��Һ�Ϳɽ�������III����Ϊ������VII�����������Բ��������������� ��
��3��������VII�ж���ͬ���칹�壬��д��һ�ַ�������Ҫ��Ľṹ��ʽ ��
i��������������ȡ����
ii��1 mol �����ʷ���������Ӧ������4 mol Ag
��4����Ӧ�Ļ�ѧ����ʽΪ ����ע��������
��5�������� �뻯����
�뻯���� ��һ�������°����ʵ���֮��1��2�ɷ������Ʒ�Ӧ�ٵķ�Ӧ����д�������Ľṹ��ʽ ��
��һ�������°����ʵ���֮��1��2�ɷ������Ʒ�Ӧ�ٵķ�Ӧ����д�������Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com