
һ�������£�ͨ�����з�Ӧ��ʵ��ȼú��������Ļ��գ�
SO2(g)��2CO(g) 2CO2(g)��S(l)����H��0
2CO2(g)��S(l)����H��0
����Ӧ�ں��ݵ��ܱ������н��У������й�˵����ȷ����(����)
A��ƽ��ǰ�����ŷ�Ӧ�Ľ��У�������ѹǿʼ�ղ���
B��ƽ��ʱ�������������䣬�����������Ӧ���ʼӿ�
C��ƽ��ʱ�������������䣬�����¶ȿ����SO2��ת����
D�������������䣬ʹ�ò�ͬ����,�÷�Ӧ��ƽ�ⳣ������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ܵĴ洢������Ӧ�õ���Ҫƿ����Ŀǰ�����û������о�����Ҫ��������У���λ�⻯��������廯���̼�ʲ��ϡ������⻯��ȡ�
(1) Ti(BH4)2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ�
��Ti2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ________��
 ��BH
��BH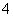 �Ŀռ乹����________(����������)��
�Ŀռ乹����________(����������)��
(2) Һ���Ǹ������ʣ������ܵ������� �壬����N2��3H2
�壬����N2��3H2
 2NH3ʵ�ִ�������⡣����˵����ȷ����________(����ѡ��)��
2NH3ʵ�ִ�������⡣����˵����ȷ����________(����ѡ��)��
A�� NH3������Nԭ�Ӳ���sp3�ӻ�
B�� ��ͬѹǿʱ��NH3�е��PH3��
C��[Cu (NH3)4]2�������У�Nԭ������λԭ��
D��CN���ĵ���ʽΪ[:C����N:]��
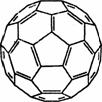 (3) 2008�꣬Yoon���˷���Ca��C60(���ӽṹ����ͼ��)���ɵ�Ca32C60��
(3) 2008�꣬Yoon���˷���Ca��C60(���ӽṹ����ͼ��)���ɵ�Ca32C60�� ��������H2���ӡ�
��������H2���ӡ�

��C60���� �����ڱ���CS2��C60��________����(����ԡ��Ǽ��ԡ�)��
�����ڱ���CS2��C60��________����(����ԡ��Ǽ��ԡ�)��
��1 mol C60�����У����ЦҼ���ĿΪ________��
(4) MgH2�ǽ����⻯�ﴢ����ϣ��侧���ṹ����ͼ����ʾ����֪�þ�����ܶ�Ϊa g��cm��3���������Ϊ________cm3[a��NA��ʾ(NA��ʾ�����ӵ�������ֵ)]��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij������Һ�����ܺ���NH4����Mg2+��Al3+��Na����Cu2����OH����Cl����I����
NO3����SO42���������еļ��֣��ֽ�������ʵ�飺
�����Թ���ȡ������Һ���μӼ���ʯ����Һ����Һ�ʺ�ɫ��
����ȡԭ��Һ��������������CCl4���ڲ����������¼���������ˮ�����ã��ϲ�ӽ���ɫ���²���Ϻ�ɫ��
���������ˮ��Һ�м���AgNO3��Һ���а�ɫ�������ɡ�
����ȡԭ��Һ���μ�NaOH��Һ���ð�ɫ��������������NaOH��Һ�����������������ܽ⣬���˺����Һ���ȣ��д̼�����ζ�����������
��1���ɴ��ж�ԭ��Һ��һ���д����� ���ӡ�
��2������ڷ�Ӧ�����ӷ���ʽ��________________________________
��3��������г����ܽ�����ӷ���ʽ��
��4��������ȷ������������ ����μ��� ��д��ʵ�����Ƽ��ж����ݵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾװ������ͭ��Ũ���ᷴӦ����ʵ�顣
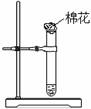 ��1�����Լ�ƿ��ȡ��һС��ͭƬ�����Թ��У����õ������� ��
��1�����Լ�ƿ��ȡ��һС��ͭƬ�����Թ��У����õ������� ��
��2����Ӧպ ��Һ���ѧʽ����
��3���Թ��г�����Һ�����ɫ�⣬������ֵ������� ������ţ���
A���к���ɫ�������ɣ�������ɫ��ͬ
B���к���ɫ�������ɣ��ϲ���ɫ��dz
C���к���ɫ�������ɣ��²���ɫ��dz
��4��ʵ��������Թ��ڲ�����Ĵ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A(g)��B(g) C(g)��D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
C(g)��D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
| �¶�/�� | 700 | 800 | 830 | 1 000 | 1 200 |
| ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
�ش��������⣺
(1)�÷�Ӧ��ƽ�ⳣ������ʽK��________����H________0(�<������>������)��
(2)830 ��ʱ����һ��5 L���ܱ������г���0.20 mol ��A��0.80 mol ��B���練Ӧ��ʼ6 s��A��ƽ����Ӧ����v(A)��0.003 mol·L��1·s��1����6 sʱc(A)��________ mol·L��1��C�����ʵ���Ϊ________mol������Ӧ��һ ��ʱ��ﵽƽ��ʱA��ת����Ϊ________�������ʱ����ܱ��������ٳ���1 mol �����ƽ��ʱA��ת����Ϊ________��
��ʱ��ﵽƽ��ʱA��ת����Ϊ________�������ʱ����ܱ��������ٳ���1 mol �����ƽ��ʱA��ת����Ϊ________��
(3)�жϸ÷�Ӧ�Ƿ�ﵽƽ�������Ϊ________(����ȷѡ��ǰ����ĸ)��
a��ѹǿ����ʱ��ı�
b��������ܶȲ���ʱ��ı�
c��c(A)����ʱ��ı�
d����λʱ��������C��D�����ʵ������
(4)1 200 ��ʱ��ӦC(g)��D(g) A(g)��B(g)��ƽ�ⳣ����ֵΪ________��
A(g)��B(g)��ƽ�ⳣ����ֵΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������OH������ �·���ˮ�ⷴӦ��
�·���ˮ�ⷴӦ��
O2NC6H4COOC2H5��OH�� O2NC6H4COO����C2H5OH
O2NC6H4COO����C2H5OH
���ַ� Ӧ��ij�ʼŨ�Ⱦ�Ϊ0.050 mol·L��1��15 ��ʱ���O2NC6H4COOC2H5��ת��������ʱ��仯�����������ʾ���ش��������⣺
Ӧ��ij�ʼŨ�Ⱦ�Ϊ0.050 mol·L��1��15 ��ʱ���O2NC6H4COOC2H5��ת��������ʱ��仯�����������ʾ���ش��������⣺
| t/s | 0 | 120 | 180 | 240 | 330 | 530 | 600 | 700 | 800 |
| ��/% | 0 | 33.0 | 41.8 | 48.8 | 58.0 | 69.0 | 70.4 | 71.0 | 71.0 |
(1)��ʽ����÷�Ӧ��120��180 s��180��240 s �����ƽ����Ӧ����________��________���Ƚ����ߴ�С�ɵó��Ľ�����____________________��
(2)��ʽ����15 ��ʱ�÷�Ӧ��ƽ�ⳣ��________��
(3)Ϊ���O2NC6H4COOC2H5��ƽ��ת���ʣ������ʵ����Ʒ�Ӧ�¶��⣬���ɲ�ȡ�Ĵ�ʩ��________(Ҫ��д������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�о����������������ڴ����к������ӵ������ʱ���漰���·�Ӧ��
2NO2(g)��NaCl(s) NaNO3(s)��ClNO(g)��K1����H1<0(��)
NaNO3(s)��ClNO(g)��K1����H1<0(��)
2NO(g)��Cl2(g) 2ClNO(g)��K2����H2<0(��)
2ClNO(g)��K2����H2<0(��)
(1)4NO2(g)��2NaCl(s) 2NaNO3(s)��2NO(g)��Cl2(g)��ƽ�ⳣ��K��________(��K1��K2��ʾ)��
2NaNO3(s)��2NO(g)��Cl2(g)��ƽ�ⳣ��K��________(��K1��K2��ʾ)��
(2)Ϊ�о���ͬ�����Է�Ӧ(��)��Ӱ�죬�ں��������£���2 L�����ܱ������м���0.2 mol NO��0.1 mol Cl2��10 m inʱ��Ӧ(��)�ﵽƽ�⡣���10 min��v(ClNO)��7.5��10��3 mol·L��1·min��1����ƽ���n(Cl2)��________mol��NO��ת������1��________�������������ֲ��䣬��Ӧ(��)�ں�ѹ�����½��У�ƽ��ʱNO��ת���ʦ�2________��1(�>����<������)��ƽ�ⳣ��K2______(�������С�����䡱)����ҪʹK2��С���ɲ�ȡ�Ĵ�ʩ��________��
inʱ��Ӧ(��)�ﵽƽ�⡣���10 min��v(ClNO)��7.5��10��3 mol·L��1·min��1����ƽ���n(Cl2)��________mol��NO��ת������1��________�������������ֲ��䣬��Ӧ(��)�ں�ѹ�����½��У�ƽ��ʱNO��ת���ʦ�2________��1(�>����<������)��ƽ�ⳣ��K2______(�������С�����䡱)����ҪʹK2��С���ɲ�ȡ�Ĵ�ʩ��________��
(3)ʵ���ҿ���NaOH��Һ����NO2����ӦΪ2NO2��2NaOH===NaNO3��NaNO2��H2O����0.2 mol NaOH��ˮ��Һ��0. 2 mol NO2ǡ����ȫ��Ӧ��1 L��ҺA����ҺBΪ0.1 mol·L��1��CH3COONa��Һ��������Һ��c(NO
2 mol NO2ǡ����ȫ��Ӧ��1 L��ҺA����ҺBΪ0.1 mol·L��1��CH3COONa��Һ��������Һ��c(NO )��c(NO
)��c(NO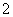 )��c(CH3COO��)�ɴ�С��˳��Ϊ____________________________________________��(��֪HNO2���볣��Ka��7.1��10��4 mol·L��1��CH3COOH�ĵ��볣��Ka��1.7��10��5 mol·L��1)
)��c(CH3COO��)�ɴ�С��˳��Ϊ____________________________________________��(��֪HNO2���볣��Ka��7.1��10��4 mol·L��1��CH3COOH�ĵ��볣��Ka��1.7��10��5 mol·L��1)
��ʹ��ҺA����ҺB��pH��ȵķ�����________��
a������ҺA�м�����ˮ
b������ҺA�м�����NaOH
c������ҺB�м�����ˮ
d������ҺB�м�����NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ʼ��仯�����ڹ�ҵ���й㷺Ӧ�á�
��1��ͬ��ʯ�ڸ������Ʊ������Ȼ�ѧ����ʽΪ��
4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g) H
H
��֪��ͬ�����£�
4Ca3(PO4)2F(s)+3SiO2(s)=6Ca3(PO4)2(s)+2CaSiO3(s)+SiF4(g) ��H1
2Ca3(PO4)2(s)+10C(s)=P4(g)+6CaO(s)+10CO(g) ��H2
SiO2(s)+CaO(s)=CaSiO3(s) ��H3
�á�H1����H2�͡�H3��ʾ H����
H���� H=____________________��
H=____________________��
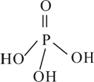
��2�������������Ϊ����������ӣ�����ṹʽ��ͼ��֮����ȥ����ˮ���Ӳ����ṹʽΪ________________________________�����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ____________��
��3���������ƣ�NaH2PO2�������ڹ�ҵ�ϵĻ�ѧ������
�ٻ�ѧ��������Һ�к���Ni2+��H2PO2���������Ե������·���������Ӧ��
��a��_____ Ni2+ + ____ H2PO2��+ _____ �� ___Ni++______ H2PO3��+ ____
��b��6H2PO-2 +2H+ =2P+4H2PO3+3H2��
���������д������ƽ��Ӧʽ��a����
�����â��з�Ӧ�������϶Ƽ����������—�Ͻ𣬴Ӷ��ﵽ��ѧ������Ŀ�ģ�����һ�ֳ����Ļ�ѧ�ơ�������·���Ƚϻ�ѧ�����ơ�
�����ϵIJ�ͬ�㣺______________________________________________________��
ԭ���ϵIJ�ͬ�㣺______________________________________________________��
��ѧ�Ƶ��ŵ㣺________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com