
·ÖĪö £Ø1£©12.4æĖNa2XÖŠŗ¬ÓŠ0.4molNa+£¬øł¾ŻNa2XµÄ×é³É¼ĘĖć³öNa2XµÄĪļÖŹµÄĮ漰Ħ¶ūÖŹĮ棬øł¾ŻÄ¦¶ūÖŹĮæÓėĻą¶Ō·Ö×ÓĮæµÄ¹ŲĻµ¼ĘĖć³öXµÄĻą¶ŌŌ×ÓÖŹĮ棬øł¾ŻNa2XĻą¶ŌŌ×ÓÖŹĮæĄ“Č·¶Ø·Ö×ÓŹ½£»
£Ø2£©Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬ĆܶČÖ®±ČµČÓŚĻą¶Ō·Ö×ÓÖŹĮæÖ®±Č£¬¾Ż“Ė¼ĘĖćH2XµÄĻą¶Ō·Ö×ÓÖŹĮ棬½ų¶ų¼ĘĖćXµÄĻą¶ŌŌ×ÓÖŹĮ棻
£Ø3£©¢ŁĶ¬ĪĀĶ¬Ń¹ĻĀ£¬ĘųĢåĢå»żÖ®±ČµČÓŚĪļÖŹµÄĮæÖ®±Č£¬½įŗĻn=$\frac{m}{M}$ÅŠ¶ĻĢå»żÖ®±Č£»Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬ĘųĢåĆܶČÖ®±ČµČÓŚĘäĻą¶Ō·Ö×ÓÖŹĮæÖ®±Č£»
¢ŚĶ¬ĪĀĶ¬Ń¹ĻĀ£¬ĘųĢåĢå»żÖ®±ČµČÓŚĪļÖŹµÄĮæÖ®±Č£¬½įŗĻm=nMÅŠ¶Ļ¶žÕßÖŹĮæÖ®±Č£®
½ā“š ½ā£ŗ£Ø1£©12.4æĖNa2XÖŠŗ¬ÓŠ0.4molNa+£¬Na2XµÄĪļÖŹµÄĮæĪŖ£ŗn£ØNa2X£©=$\frac{1}{2}$n£ØNa+£©=0.4mol”Į$\frac{1}{2}$=0.2mol£¬
Na2XµÄĦ¶ūÖŹĮæĪŖ£ŗM£ØNa2X£©=$\frac{12.4g}{0.2mol}$=62g/mol£»
Ħ¶ūÖŹĮæŌŚŹżÖµÉĻµČÓŚĘäĻą¶Ō·Ö×ÓÖŹĮ棬¼“Na2XµÄĻą¶ŌŌ×ÓĮæĪŖ62£»
ÄĘŌ×ÓµÄĻą¶ŌŌ×ÓÖŹĮæŹĒ23£¬ĖłŅŌXµÄĻą¶ŌŌ×ÓÖŹĮæŹĒ62-23”Į2=16£»
XĪŖŃõŌ×Ó£¬øĆĪļÖŹµÄ»ÆѧŹ½ĪŖNa2O£»
¹Ź“š°øĪŖ£ŗ62g/mol£»62£»16£»Na2O£»
£Ø2£©ĻąĶ¬×“æöĻĀ£¬Ņ»¶ØĢå»żµÄĘųĢ¬Ēā»ÆĪļHmXµÄÖŹĮæŹĒµČĢå»żNH3µÄ2±¶£¬Ōņ¶žÕßĆܶČÖ®±ČĪŖ2£ŗ1£¬Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬ĆܶČÖ®±ČµČÓŚĻą¶Ō·Ö×ÓÖŹĮæÖ®±Č£¬HmXµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ17”Į2=34£¬ŌņXµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ34-m£¬
¹Ź“š°øĪŖ£ŗ34-m£»
£Ø3£©¢Łøł¾Żn=$\frac{m}{M}$æÉÖŖ£¬Ķ¬ÖŹĮæµÄX”¢YĘųĢåĪļÖŹµÄĮæÖ®±ČÓėĦ¶ūÖŹĮæ³É·“±Č£¬¼“Ķ¬ÖŹĮæµÄX”¢YĘųĢåĪļÖŹµÄĮæÖ®±Č=B£ŗA£¬Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬ĘųĢåĢå»żÖ®±ČµČÓŚĪļÖŹµÄĮæÖ®±Č=B£ŗA£»
Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬ĘųĢåĆܶČÖ®±ČµČÓŚĘäĻą¶Ō·Ö×ÓÖŹĮæÖ®±Č=A£ŗB£»
¹Ź“š°øĪŖ£ŗB£ŗA£»B£ŗA£»A£ŗB£»
¢ŚĶ¬ĪĀĶ¬Ń¹ĻĀ£¬ĪļÖŹµÄĮæÖ®±ČµČÓŚĢå»żÖ®±Č£¬¹ŹĻąĶ¬Ģå»żµÄX”¢YµÄĪļÖŹµÄĮæÖ®±Č=1£ŗ1£¬½įŗĻm=nMæÉÖŖ£¬¶žÕßÖŹĮæÖ®±Č=Ħ¶ūÖŹĮæÖ®±Č=A£ŗB£¬
¹Ź“š°øĪŖ£ŗA£ŗB£»1£ŗ1£®
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹµÄĮæµÄ¼ĘĖć£¬ĢāÄæÄŃ¶Č²»“ó£¬Ć÷Č·ĪļÖŹµÄĮæÓėĦ¶ūÖŹĮ攢°¢·üŁ¤µĀĀŽ³£ŹżÖ®¼äµÄ¹ŲĻµĪŖ½ā“š¹Ų¼ü£¬ŹŌĢāÓŠĄūÓŚĢįøßѧɜµÄ·ÖĪöÄÜĮ¦¼°»Æѧ¼ĘĖćÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 56gĢśøßĪĀĻĀÓė×ćĮæĖ®ÕōĘų³ä·Ö·“Ó¦×ŖŅʵē×ÓŹżÄæĪŖ3NA | |
| B£® | 100 g CaCO3ŗĶKHCO3»ģŗĻ¹ĢĢåÖŠCO32-µÄŹżÄæĪŖNA | |
| C£® | ŹµŃéŹŅÓĆH2O2Öʱø1mol O2×ŖŅʵĵē×ÓŹżĪŖ2NA | |
| D£® | ±ź×¼×“æöĻĀ£¬2.24L CCl4ŗ¬ÓŠµÄŌ×ÓŹżĪŖ0.5NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 ½«½ą¾»µÄ½šŹōʬ¼×”¢ŅŅ”¢±ū”¢¶”·Ö±š·ÅÖĆŌŚ½žÓŠÄ³ÖÖŃĪČÜŅŗµÄĀĖÖ½ÉĻĆę²¢Ń¹½ō£ØČēĶ¼ĖłŹ¾£©£®ŌŚĆæ“ĪŹµŃ鏱£¬¼ĒĀ¼µēŃ¹ÖøÕėµÄŅĘ¶Æ·½ĻņŗĶµēŃ¹±ķµÄ¶ĮŹżČē±ķ
½«½ą¾»µÄ½šŹōʬ¼×”¢ŅŅ”¢±ū”¢¶”·Ö±š·ÅÖĆŌŚ½žÓŠÄ³ÖÖŃĪČÜŅŗµÄĀĖÖ½ÉĻĆę²¢Ń¹½ō£ØČēĶ¼ĖłŹ¾£©£®ŌŚĆæ“ĪŹµŃ鏱£¬¼ĒĀ¼µēŃ¹ÖøÕėµÄŅĘ¶Æ·½ĻņŗĶµēŃ¹±ķµÄ¶ĮŹżČē±ķ | ½šŹō | µē×ÓĮ÷¶Æ·½Ļņ | µēŃ¹ |
| ¼× | ¼×”śCu | +0.78 |
| ŅŅ | Cu”śŅŅ | +0.15 |
| ±ū | ±ū”śCu | +1.35 |
| ¶” | ¶””śCu | +0.30 |
| A£® | ŌŚĖÄÖÖ½šŹōÖŠŅŅµÄ»¹ŌŠŌ×īĒæ | |
| B£® | ½šŹōŅŅÄÜ“ÓĮņĖįĶČÜŅŗÖŠÖĆ»»³öĶ | |
| C£® | ¼×”¢¶”ČōŠĪ³ÉŌµē³ŲŹ±£¬¼×ĪŖøŗ¼« | |
| D£® | ¼×”¢ŅŅŠĪ³ÉŗĻ½šŌŚæÕĘųÖŠ£¬ŅŅĻȱ»øÆŹ“ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ś¢Ż | B£® | ¢Ś¢Ž¢ą | C£® | ¢Ś¢Ū¢Ż¢ß¢ą | D£® | ¢Ł¢Ś¢Ü¢Ż¢ą |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
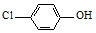 £©µÄ·ĻĖ®æÉŅŌĄūÓĆĪ¢ÉśĪļµē³Ų³żČ„£¬ĘäŌĄķČēĶ¼1ĖłŹ¾£®
£©µÄ·ĻĖ®æÉŅŌĄūÓĆĪ¢ÉśĪļµē³Ų³żČ„£¬ĘäŌĄķČēĶ¼1ĖłŹ¾£®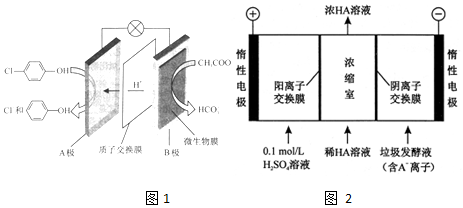
 -OH+2e-+H+ØT
-OH+2e-+H+ØT -OH+Cl-
-OH+Cl-²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
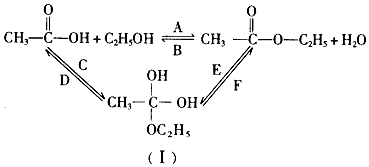
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ņ»¶ØĢõ¼žĻĀ£¬2molSO2 ŗĶ 1molO2 »ģŗĻŌŚĆܱÕČŻĘ÷ÖŠ³ä·Ö·“Ó¦ŗóČŻĘ÷ÖŠµÄ·Ö×ÓŹż“óÓŚ2NA | |
| B£® | 256g S8 ·Ö×ÓÖŠŗ¬ S-S ¼üĪŖ 7NA øö | |
| C£® | ÓÉ 1molCH3COONa ŗĶÉŁĮæ CH3COOH ŠĪ³ÉµÄÖŠŠŌČÜŅŗÖŠ£¬CH3COO-ŹżÄæĪŖ NA øö | |
| D£® | 1 mol Na Óė O2 ĶźČ«·“Ó¦£¬Éś³É NaO2 ŗĶ Na2O2µÄ»ģŗĻĪļ£¬×ŖŅʵē×Ó×ÜŹżĪŖ NA øö |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com