
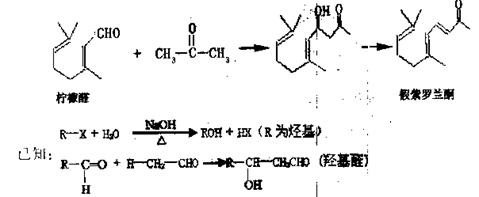
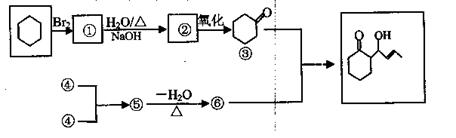

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
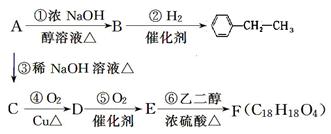
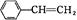 “ó36.5£¬Ēė»Ų“š£ŗ
“ó36.5£¬Ēė»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
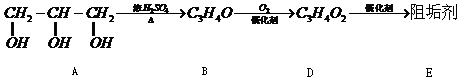

 Ė®½āÖĘµĆ£ØĢī”°ĢĒĄą”±”¢”°ÓĶÖ¬”±»ņ”°µ°°×ÖŹ”±£©
Ė®½āÖĘµĆ£ØĢī”°ĢĒĄą”±”¢”°ÓĶÖ¬”±»ņ”°µ°°×ÖŹ”±£©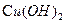 ·“Ӧɜ³ÉD£¬Ęä»Æѧ·½³ĢŹ½ĪŖ ”£
·“Ӧɜ³ÉD£¬Ęä»Æѧ·½³ĢŹ½ĪŖ ”£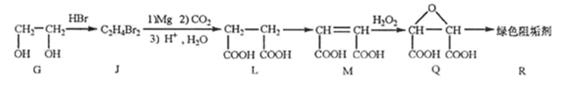
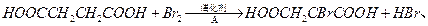
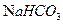 ČÜŅŗ×÷ÓĆ²śÉś2mol
ČÜŅŗ×÷ÓĆ²śÉś2mol 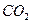 £¬TµÄ½į¹¹¼ņŹ½ĪŖ £ØÖ»Š“Ņ»ÖÖ£©”£
£¬TµÄ½į¹¹¼ņŹ½ĪŖ £ØÖ»Š“Ņ»ÖÖ£©”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
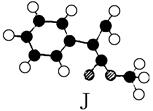
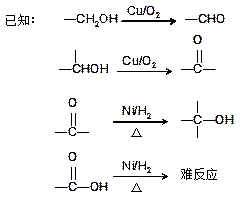
| A£®ÄÜÓėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·¢Éś¼Ó³É·“Ó¦ | B£®²»ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«? |
| C£®ŌŚ¼īŠŌĢõ¼žĻĀÄÜ·¢ÉśĖ®½ā·“Ó¦ | D£®²»æÉÄÜ·¢Éś¼Ó¾Ū·“Ó¦? |
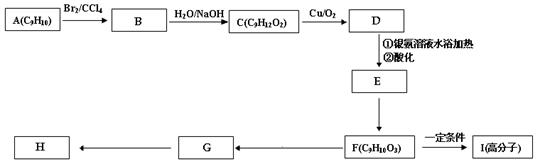
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 £ØĘäÖŠI”¢IIĪŖĪ“ÖŖ²æ·ÖµÄ½į¹¹£©£¬ĪŖĶĘ²āXµÄ·Ö×Ó½į¹¹£¬½ųŠŠČēĻĀĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ż³Ģ£ŗ
£ØĘäÖŠI”¢IIĪŖĪ“ÖŖ²æ·ÖµÄ½į¹¹£©£¬ĪŖĶĘ²āXµÄ·Ö×Ó½į¹¹£¬½ųŠŠČēĻĀĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ż³Ģ£ŗ
 Ņ»ÖÖŗ¬Ńõ¹ŁÄÜĶŵÄĆū³ĘŹĒ£ŗ £»XµÄ½į¹¹¼ņŹ½ĪŖ£ŗ £»
Ņ»ÖÖŗ¬Ńõ¹ŁÄÜĶŵÄĆū³ĘŹĒ£ŗ £»XµÄ½į¹¹¼ņŹ½ĪŖ£ŗ £»| A£®¼Ó³É·“Ó¦ | B£®ĻūČ„·“Ó¦ | C£®Č”“ś·“Ó¦ | D£®Ńõ»Æ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

 £Øø±²śĪļ¾łĪ“Š“³ö£©”£
£Øø±²śĪļ¾łĪ“Š“³ö£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
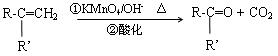 £ØR”¢R”ƱķŹ¾Ģž»ł»ņ¹ŁÄÜĶÅ£©
£ØR”¢R”ƱķŹ¾Ģž»ł»ņ¹ŁÄÜĶÅ£©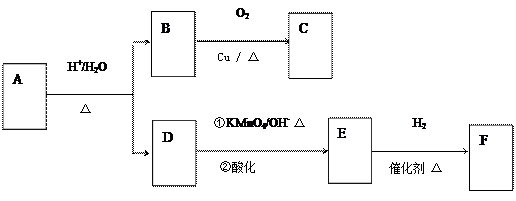
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
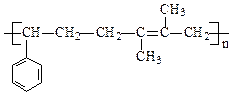
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
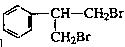
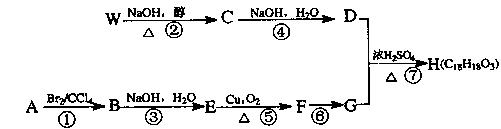
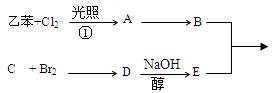
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com