
 £¬¶ų²»ÅŲ¼³É
£¬¶ų²»ÅŲ¼³É £¬ĘäÖ±½ÓµÄøł¾ŻŹĒŗéĢŲ¹ęŌņ£®
£¬ĘäÖ±½ÓµÄøł¾ŻŹĒŗéĢŲ¹ęŌņ£® ·ÖĪö £Ø1£©3d¹ģµĄ°ė³äĀśĖµĆ÷3d¹ģµĄÖŠŗ¬ÓŠ5øöµē×Ó£¬øł¾Żµē×ÓÅŲ¼¹ęŌņÖŖ£¬øĆŌ×ÓµÄ4sÄܼ¶ÉĻµē×ÓČ«Āś»ņ°ė³äĀś£¬½įŗĻ¹¹ŌģŌĄķĄ“·ÖĪö½ā“š£»
£Ø2£©øł¾Ż¼¤·¢Ģ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½Č·¶ØøĆŌ×ÓŗĖĶāµē×ÓŹż£¬Ō×ÓÖŠŗĖĶāµē×ÓŹż=Ō×ÓŠņŹż£¬“Ó¶ųČ·¶ØøĆŌŖĖŲ£¬øł¾Ż¹¹ŌģŌĄķŠ“³öøĆŌŖĖŲ»łĢ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½£»
£Ø3£©ŗéĢŲ¹ęŌņŅŖĒóµē×ÓÓÅĻČÕ¼¾ŻŅ»øöæÕ¹ģµĄ£¬²¢ĒŅŌŚŅ»øö¹ģµĄÖŠ×ŌŠż·½ĻņĻą·“£®
½ā“š ½ā£ŗ£Ø1£©3d¹ģµĄ°ė³äĀśĖµĆ÷3d¹ģµĄÖŠŗ¬ÓŠ5øöµē×Ó£¬øł¾Żµē×ÓÅŲ¼¹ęŌņÖŖ£¬øĆŌ×ÓµÄ4sÄܼ¶ÉĻµē×ÓČ«Āś»ņ°ė³äĀś£¬ĖłŅŌøĆ»łĢ¬Ō×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ£ŗ[Ar]3d54s1»ņ[Ar]3d54s2£¬ĖłŅŌĪŖCrŗĶMn£¬ŌņŌ×ÓŠņŹż±Č½ĻŠ”µÄCrŌŖĖŲ»łĢ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ[Ar]3d54s1£»ÓŠ6øöĪ“³É¶Ōµē×Ó£»
¹Ź“š°øĪŖ£ŗCr£»Mn£»[Ar]3d54s1£»6£»
£Ø2£©øł¾Ż¼¤·¢Ģ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ÖŖøĆŌŖĖŲŗĖĶāÓŠ16øöµē×Ó£¬øł¾Ż¹¹ŌģŌĄķÖŖ£¬Ę仳Ģ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ£ŗ1s22s22p63s23p4£¬¹Ź“š°øĪŖ£ŗ1s22s22p63s23p4£»
£Ø3£©ŅņĪŖŗéĢŲ¹ęŌņŅŖĒóµē×ÓÓÅĻČÕ¼¾ŻŅ»øöæÕ¹ģµĄ£¬²¢ĒŅŌŚŅ»øö¹ģµĄÖŠ×ŌŠż·½ĻņĻą·“£¬ĖłŅŌŌŚd¹ģµĄÖŠµē×ÓÅŲ¼³É £¬¶ų²»ÅŲ¼³É
£¬¶ų²»ÅŲ¼³É £¬ĘäÖ±½ÓµÄøł¾ŻŹĒŗéĢŲ¹ęŌņ£¬¹Ź“š°øĪŖ£ŗŗéĢŲ¹ęŌņ£»
£¬ĘäÖ±½ÓµÄøł¾ŻŹĒŗéĢŲ¹ęŌņ£¬¹Ź“š°øĪŖ£ŗŗéĢŲ¹ęŌņ£»
µćĘĄ ±¾Ģāæ¼²éĮĖŌŖĖŲŗĖĶāµē×ÓÅŲ¼£¬Ć÷Č·µē×ÓÅŲ¼¹ęŌņ¼“¹¹ŌģŌĄķŹĒ½ā±¾Ģā¹Ų¼ü£¬×¢ŅāŌ×Ó¹ģµĄÖŠµē×Ó“¦ÓŚČ«æÕ”¢°ėĀś”¢Č«ĀśŹ±×īĪČ¶Ø£¬ÄѶČÖŠµČ£®


 ³å“Ģ100·Ö1ŗžķĻµĮŠ“š°ø
³å“Ģ100·Ö1ŗžķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A | B | C | D | |
| µē½āÖŹČÜŅŗ | HCl | AgNO3 | BaCl2 | KOH |
| pHÖµ±ä»Æ | ¼õŠ” | Ōö“ó | ±ä“ó | ²»±ä |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH3µÄ°Ł·Öŗ¬Įæ²»±ä | B£® | N2µÄĢå»ż·ÖŹżŌö“ó | ||
| C£® | N2µÄ×Ŗ»ÆĀŹŌö“ó | D£® | NH3µÄ°Ł·Öŗ¬ĮæŌöŠ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
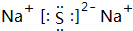 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻņĢś·ŪÖŠ¼ÓČėĻ”ĻõĖį | |
| B£® | ĻņÉÕ¼īČÜŅŗÖŠĶØČėCO2ĘųĢå | |
| C£® | ĻņĻ”ŃĪĖįÖŠµĪČėÉŁĮæµÄNaAlO2ČÜŅŗ | |
| D£® | ĻņMgSO4”¢H2SO4µÄ»ģŗĻČÜŅŗÖŠ¼ÓČė¹żĮæµÄBa£ØOH£©2ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé | ŹµŃéÄæµÄ |
| A | ÄĘŗĶĆ¾·Ö±šĶ¶ČėĄäĖ®ÖŠ | ÅŠ¶ĻÄĘŗĶĆ¾½šŹōŠŌĒæČõ |
| B | ŌŚMgCl2ÓėAlCl3ČÜŅŗÖŠ·Ö±š¼ÓČė¹żĮæµÄ°±Ė® | ÅŠ¶ĻĆ¾ÓėĀĮµÄ½šŹōŠŌĒæČõ |
| C | Ļņ¹čĖįÄĘČÜŅŗÖŠĶØČėCO2 | ÅŠ¶ĻĢ¼ĖįÓė¹čĖįµÄĖįŠŌĒæČõ |
| D | Br2ÓėI2·Ö±šÓė×ćĮæµÄH2·“Ó¦ | ÅŠ¶ĻäåÓėµāµÄ·Ē½šŹōŠŌĒæČõ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
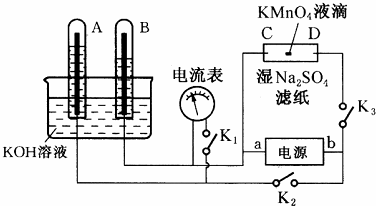
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
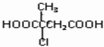 µÄŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼£ØĪŽ»śŹŌ¼ĮČĪŃ”£©£®ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ź¾ĄżČēĻĀ£ŗ£ØRCH3CH=CH2+HCl$\stackrel{“߻ƼĮ}{”ś}$RCH3CHClCH3£©
µÄŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼£ØĪŽ»śŹŌ¼ĮČĪŃ”£©£®ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ź¾ĄżČēĻĀ£ŗ£ØRCH3CH=CH2+HCl$\stackrel{“߻ƼĮ}{”ś}$RCH3CHClCH3£©  £®
£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com