
a��CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g) ��H1��+256.6 kJ��mol��1
b��2CH3CH2OH(g)��O2(g)��6H2(g)��4CO(g) ��H2��+27.6 kJ��mol��1
������˵����ȷ����
A. ����a�ķ�Ӧ�¶ȣ��Ҵ���ת��������
B. ��b��֪���Ҵ���ȼ����Ϊ13.8 kJ��mol��1
C. 2H2(g)��O2(g)��2H2O(g) ��H����485.6 kJ��mol��1
D. ��ȡ������������;��b���ĵ���������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�γ��и߶�ѧҵˮƽģ�⣨һ����ѧ�Ծ��������棩 ���ͣ�ѡ����
�ҹ��ɵij����ߺ����ػ������Һ����ú����Ϊ�ƽ�����ú������(����)

A. ������ B. ����� C. ������ D. ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�����и�����һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
��ʽ������þ[Mga(ClO)b(OH)c��xH2O]��һ���п�����ֵ������ˮ������������Ϊȷ����ʽ������þ����ɣ���������ʵ�飺
�� ȷ��ȡ1.685g��ʽ������þ������250mL��ƿ�У����������KI��Һ�������������ữ����O.8000mol/LNa2S2O3����Һ�ζ����յ㣨���ӷ���ʽΪ2S2O32-��I2=2I-��S4O62-��������25.00mL��
�� ��ȡ1.685g��ʽ������þ�����������������ữ����������3%H2O2��Һ���������ٲ�������(H2O2��ClO-����ΪO2)��ϡ����1000mL����ȡ25.00mL��Һ����ƿ�У���һ����������0.020 00mol/L EDTA(Na2H2Y)����Һ�ζ����е�Mg2+�����ӷ���ʽΪMg2++H2Y2-=MgY2-+2H+) ������25.00 mL
��1������� ����Ҫ�õ���ָʾ����_______��
��2������� �����ζ�����ʹ��ǰδ��EDTA����Һ��ϴ����õ�Mg2+���ʵ�����____���ƫ�ߡ�����ƫ�͡����䡱����
��3��ͨ������ȷ����ʽ������þ�Ļ�ѧʽ��д��������̣���____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�����и�����һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ��������������������ء������й�˵���������
A. �Ӻ�ˮ�п���ȡþ����������Ȼ�þ���Ƶý���þ
B. Ӧ�øߴ��ȵ��ʹ��Ƴɹ��ά�������Ϣ�����ٶ�
C. ���Ƹ����ܵ���ĥ��̥���ɼ���PM2.5��ϸ������IJ���
D. ����������̼�Ƴɵ�ȫ�������ϣ������������صġ���ɫ��Ⱦ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ�����и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�˵������ȷ����
A. ��֪25��ʱNH4CN��Һ�Լ���,��25��ʱ�ĵ���ƽ�ⳣ��K(NH3��H2O)>K(HCN)
B. ��ˮ�������c(H��)=10��12mol��L��1����Һ��:Na����Ba������HCO3����Cl�����Դ�������
C. ��֪Ksp(AgCl)=1.56��10��10�� Ksp(Ag2CrO4)=9.0��10��12������Cl����CrO4������Ũ�Ⱦ�Ϊ0.010 mol��L-1��Һ����μ���0.010 mol��L-1��AgNO3��Һʱ��CrO4�����Ȳ�������
D. ������pH=7��CH3COOH��NaOH�����Һ�У�c(Na��)>c(CH3COO��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ�����и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��NAΪ�����ӵ�������ֵ������˵����ȷ����( )
A. 136g���ڵ�KHSO4�к���2NA��������
B. 40gH218O��40gD2O��������������Ϊ20NA
C. 1molFe�ֱ���������ϡ�����ϡ���ᷴӦת�Ƶ�������Ϊ2NA
D. ��״���£�22.4LNO��11.2LO2��Ϻ�����ķ�������ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�����и�һ��ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й���Ԫ�����ڱ���Ԫ�������ɵ�˵���������( )
A. Li��Na��KԪ�ص�ԭ�Ӻ�����Ӳ������ź˵���������Ӷ�����
B. �ڶ�����Ԫ�ش�Li��F���ǽ���������ǿ
C. ��ΪNa��K����ʧȥ���ӣ�����Na��K�Ļ�ԭ��ǿ
D. O��SΪͬ����Ԫ�أ���O��S�ķǽ�����ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�����и�һ��ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӡ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ� ��
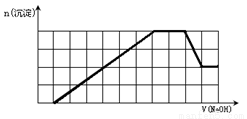
A. ԭ��Һ�к��е���������H+��NH4+��Mg2+��Al3+
B. ԭ��Һ��һ������SO42-��Na+
C. ԭ��Һ�к��е�Fe3+��Al3+�����ʵ���֮��Ϊ1��1
D. ��Ӧ����γɵ���Һ�к��е�����ΪNa2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶���ѧ��ѧҵˮƽģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
������ѧ�����������ھ�ȷ�����(In)Ԫ�ص����ԭ������Ϊ114.8����ֵ������ԭ����ίԱ��ȷ��Ϊ�±���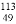 In�к��е���������
In����������
A. 49 B. 64 C. 113 D. 114.8
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com