
��8��)���ᡢ�������������ѧ�γ����ġ������ᡱ���־��������������Ӧ��������ش��������⣺
(1)ϡ�����Cu��Ӧ������ϡ�����м���H2O2����������������������ʱ��ԭ����Ϊˮ�������ʹͭ˳���ܽ⣬���õ���ɫ��Һ���÷�Ӧ�Ļ�ѧ����ʽΪ��_________________��
(2)��һ�������18 mol��L-1��Ũ�����м������ͭƬ������ʹ֮��Ӧ��������ԭ������Ϊ0.9mol����Ũ�����ʵ�����_________(����ڡ��������ڡ���С�ڡ�)100mL����ʹʣ���ͭƬ�����ܽ⣬�������м�����������Һ����KNO3��Һ������÷�Ӧ�����ӷ���ʽΪ__________________________________��
��3��������ͼ�����������ƶ���XΪ______________(�����)��
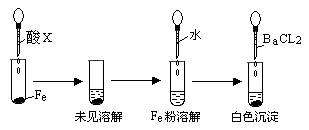
a��Ũ���� b��Ũ���� c��Ũ����
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�꽭��ʡ�����н����Ҹ�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��8��)���ᡢ�������������ѧ�γ����ġ������ᡱ���־��������������Ӧ��������ش��������⣺
(1)ϡ�����Cu��Ӧ������ϡ�����м���H2O2����������������������ʱ��ԭ����Ϊˮ�������ʹͭ˳���ܽ⣬���õ���ɫ��Һ���÷�Ӧ�Ļ�ѧ����ʽΪ��_________________��
(2)��һ�������18 mol��L-1��Ũ�����м������ͭƬ������ʹ֮��Ӧ��������ԭ������Ϊ0.9mol����Ũ�����ʵ�����_________(����ڡ��������ڡ���С�ڡ�)100mL����ʹʣ���ͭƬ�����ܽ⣬�������м�����������Һ����KNO3��Һ������÷�Ӧ�����ӷ���ʽΪ__________________________________��
��3��������ͼ�����������ƶ���XΪ______________(�����)��
a��Ũ���� b��Ũ���� c��Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ʡ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(8��)��ش����и��ʣ�
��1�������pH��Χ�� ���ڿ�����������ĸ���ָ�����п������������� ��
��2��ʯ�ҷ���ĿǰӦ����㷺�Ĺ�ҵ�����������������£�ͨ������ʯ��ʯ�õ���ʯ�ң�����ʯ��Ϊ���������������������е�SO2��Ӧ������̶���д����Ӧ�Ļ�ѧ����ʽ ��
��3������ͭ���Ʊ�Cu-Zn-Alϵ��������Ҫԭ�ϣ���ҵ����ϴ���ķ�ͭм��ԭ�����Ʊ�����ͭ�������Ʊ��������ϡ���ɫ��ѧ��˼����� ������ţ���
�� Cu + HNO3��Ũ���� Cu(NO3)2
�� Cu + HNO3��ϡ���� Cu(NO3)2
�� Cu 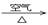 CuO
CuO Cu(NO3)2
Cu(NO3)2
��4����100mL 18mol��L-1��Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ�������������ڱ�״���µ���������� ��
A��40.32L B��30.24L C��20.16L D��13.44L
��5��ijͬѧ�����ͭƬ��ϡ�����м���H2O2��ͭƬ�ܽ⣬���Ҹ÷�Ӧ�IJ���ֻ���Ȼ�ͭ��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�����н����Ҹ�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��8��)���ᡢ�������������ѧ�γ����ġ������ᡱ���־��������������Ӧ��������ش��������⣺
(1)ϡ�����Cu��Ӧ������ϡ�����м���H2O2����������������������ʱ��ԭ����Ϊˮ�������ʹͭ˳���ܽ⣬���õ���ɫ��Һ���÷�Ӧ�Ļ�ѧ����ʽΪ��_________________��
(2)��һ�������18 mol��L-1��Ũ�����м������ͭƬ������ʹ֮��Ӧ��������ԭ������Ϊ0.9mol����Ũ�����ʵ�����_________(����ڡ��������ڡ���С�ڡ�)100mL����ʹʣ���ͭƬ�����ܽ⣬�������м�����������Һ����KNO3��Һ������÷�Ӧ�����ӷ���ʽΪ__________________________________��
��3��������ͼ�����������ƶ���XΪ______________(�����)��

a��Ũ���� b��Ũ���� c��Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8��)���ᡢ�������������ѧ�γ����ġ������ᡱ���־��������������Ӧ��������ش��������⣺
(1)ϡ�����Cu��Ӧ������ϡ�����м���H2O2����������������������ʱ��ԭ����Ϊˮ�������ʹͭ˳���ܽ⣬���õ���ɫ��Һ���÷�Ӧ�Ļ�ѧ����ʽΪ��_________________��
(2)��һ�������18 mol��L-1��Ũ�����м������ͭƬ������ʹ֮��Ӧ��������ԭ������Ϊ0.9mol����Ũ�����ʵ�����_________(����ڡ��������ڡ���С�ڡ�)100mL����ʹʣ���ͭƬ�����ܽ⣬�������м�����������Һ����KNO3��Һ������÷�Ӧ�����ӷ���ʽΪ__________________________________��
��3��������ͼ�����������ƶ���XΪ______________(�����)��

Ũ���� b��Ũ���� c��Ũ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com