��������ͭ�ĵ��ʡ��Ͻ��仯���������������е�Ӧ�������㷺��
��1����Ԫ����Ԫ�����ڱ��е�λ����
�������ڵ�VIII��
�������ڵ�VIII��
��
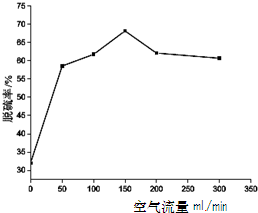
��2�������������Խ����һ����������价������ȫ���ܵ�Խ��Խ��Ĺ�ע����ԭ����ͼ��ʾ��
�ٸõ�ص��ܷ�Ӧ��ѧ����ʽΪ
4Al+3O2+6H2O=4Al��OH��3
4Al+3O2+6H2O=4Al��OH��3
��
�ڵ����NaCl��������
��ǿ��Һ�ĵ�������
��ǿ��Һ�ĵ�������
��
��3��������ص��CuSO
4��Һ���缫��Ϊ���缫����ͨ��һ��ʱ���һ����������ɫ���壬��һ���ĵ缫��ӦʽΪ
4OH--4e-=O2��+2H2O
4OH--4e-=O2��+2H2O
����ʱ����Һ�м���8g CuO������ʹ��Һ�ָ������ǰ��Ũ�ȣ�����������ռ����������ڱ�״�������Ϊ
1.12
1.12
L��
��4���Ȼ����㷺�����л��ϳɺ�ʯ��ҵ�Ĵ�������������ۻ�Ϻ���Ȳ�ͨ���������ɵõ��Ȼ�����ͬʱ����CO��д���÷�Ӧ�Ļ�ѧ����ʽ
Al
2O
3+3C+3Cl
22AlCl
3+3CO��
Al
2O
3+3C+3Cl
22AlCl
3+3CO��
��
��5��������������������ͭ��Cu
2O�����Ǻ�ɫ��ĩ�����������ϣ���֪������ͭ����ϡ��������Cu��CuSO
4��ȡ����Fe
2O
3��Cu
2O��ɵĻ�����������ϡ�����У�
�ٴ˹����з����ķ�Ӧ�У������ӷ���ʽ��ʾ����
Fe
2O
3+6H
+=2Fe
3++3H
2O��
Cu2O+2H+=Cu+Cu2++H2O
Cu2O+2H+=Cu+Cu2++H2O
��
Cu+2Fe3+=2Fe2++Cu2+
Cu+2Fe3+=2Fe2++Cu2+
�����ʵ��֤����Ӧ��������Һ����Ԫ�ؿ��ܴ�����ʽ
ȡ����������Һ���Թ��У������еμ����軯����Һ������Һ�����˵�����������ӣ���֮���ޣ���ȡ����������Һ���Թ��У������еμ����Ը��������Һ����Һ��ɫ˵�������������ӣ�����ɫ˵��û����������
ȡ����������Һ���Թ��У������еμ����軯����Һ������Һ�����˵�����������ӣ���֮���ޣ���ȡ����������Һ���Թ��У������еμ����Ը��������Һ����Һ��ɫ˵�������������ӣ�����ɫ˵��û����������
��
����ʵ���й۲쵽��Һ��Ϊ��ɫ���й���ʣ�࣬��n��Cu
2O��
��
��
n��Fe
2O
3���������������������=����

 ��У����ϵ�д�
��У����ϵ�д�
 ��������ͭ�ĵ��ʡ��Ͻ��仯���������������е�Ӧ�������㷺��
��������ͭ�ĵ��ʡ��Ͻ��仯���������������е�Ӧ�������㷺��