
ij������Һ��ֻ����NH4+��Cl-��H+��OH-4�����ӣ�����˵������ȷ����
A������pH=3��HCl��pH=11��NH3��H2O��Һ�������϶���
B������Һ�����Ӽ�һ�����㣺c��NH4+��+c��H+��= c��OH-��+c��Cl-��
C����������NH3��H2O����Һ������Ũ�ȿ���Ϊ��c��NH4+��>c��C1-��>c��OH-��>c��H+��
D������Һ�����ɵ����ʵ���Ũ�ȡ��������HCl��Һ��NH3��H2O��Һ��϶���
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л�������F�Ǻϳɵ��ӱ�Ĥ���ϸ߾���Z �����ܼ�P����Ҫԭ�ϡ�
��1��ijͬѧ���������ϩ�ϳɸ߾���Z��3��·�ߣ�I��II��III������ͼ��ʾ��
�� 3���ϳ�·���У�����Ϊ���ϡ�ԭ�Ӿ��á�Ҫ��ĺϳ�·���ǣ�����š�I������II����III���� ��
�� X�Ľṹ��ʽ�� ��
�� 1 mol F��O2�г��ȼ�գ�����7.5 mol O2������8molCO2 ��3molH2O��1molF������NaHCO3��Һ��Ӧ����2 mol CO2��������ڵ���ԭ�Ӵ���3�ֲ�ͬ�Ļ�ѧ������
F�����к��еĺ��������ŵ������� ��
Y��F��Z��Ӧ�Ļ�ѧ����ʽ�� ��
��2����֪����R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
�� A��B��Ӧ�Ļ�ѧ����ʽ�� ��
�� B��C�ķ�Ӧ�У�B�����ڼ�����������ȥһ��ˮ���ӣ�����C��C������ֻ��1��̼ԭ��������ԭ�ӡ�C�Ľṹ��ʽ�� ��
�� P�Ľṹ��ʽ�� ��
�� ��������������B��ͬ���칹�干�У������֣� �֡�
a��������������ˮ��ΪM��N b��һ��������M����ת��ΪN
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ƹ���ѧ2010�����6����Ӧ�Կ��������������Ի�ѧA �� ���ͣ������
�л�������F�Ǻϳɵ��ӱ�Ĥ���ϸ߾���Z �����ܼ�P����Ҫԭ�ϡ�
��1��ijͬѧ���������ϩ�ϳɸ߾���Z��3��·�ߣ�I��II��III������ͼ��ʾ��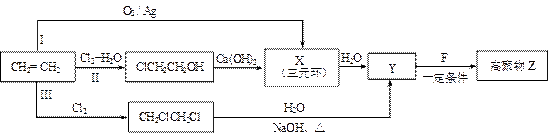
�� 3���ϳ�·���У�����Ϊ���ϡ�ԭ�Ӿ��á�Ҫ��ĺϳ�·���ǣ�����š�I������II����III���� ��
�� X�Ľṹ��ʽ�� ��
�� 1 mol F��O2�г��ȼ�գ�����7.5 mol O2������8molCO2��3molH2O��1mol F������NaHCO3��Һ��Ӧ����2 mol CO2��������ڵ���ԭ�Ӵ���3�ֲ�ͬ�Ļ�ѧ������
F�����к��еĺ��������ŵ������� ��
Y��F��Z��Ӧ�Ļ�ѧ����ʽ�� ��
��2����֪�� ��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
�� A��B��Ӧ�Ļ�ѧ����ʽ�� ��
�� B��C�ķ�Ӧ�У�B�����ڼ�����������ȥһ��ˮ���ӣ�����C��C������ֻ��1��̼ԭ��������ԭ�ӡ�C�Ľṹ��ʽ�� ��
�� P�Ľṹ��ʽ�� ��
�� ��������������B��ͬ���칹�干�У������֣� �֡�
a���������� ����ˮ��ΪM��N b��һ��������M����ת��ΪN
����ˮ��ΪM��N b��һ��������M����ת��ΪN
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ƹ���ѧ2010�����6����Ӧ�Կ��������������Ի�ѧA�� ���ͣ������
�л�������F�Ǻϳɵ��ӱ�Ĥ���ϸ߾���Z �����ܼ�P����Ҫԭ�ϡ�
��1��ijͬѧ���������ϩ�ϳɸ߾���Z��3��·�ߣ�I��II��III������ͼ��ʾ��
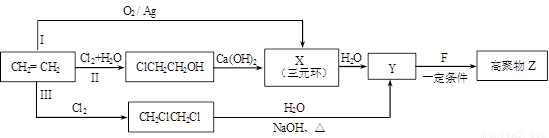
�� 3���ϳ�·���У�����Ϊ���ϡ�ԭ�Ӿ��á�Ҫ��ĺϳ�·���ǣ�����š�I������II����III���� ��
�� X�Ľṹ��ʽ�� ��
�� 1 mol F��O2�г��ȼ�գ�����7.5 mol O2������8molCO2 ��3molH2O��1mol F������NaHCO3��Һ��Ӧ����2 mol CO2��������ڵ���ԭ�Ӵ���3�ֲ�ͬ�Ļ�ѧ������
F�����к��еĺ��������ŵ������� ��
Y��F��Z��Ӧ�Ļ�ѧ����ʽ�� ��
��2����֪��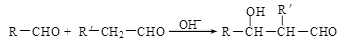 ��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
��R��R��������������ԭ�ӣ����ϳ�P��·������ͼ��ʾ��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����������CH3��
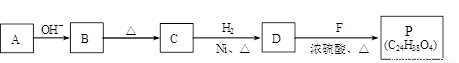
�� A��B��Ӧ�Ļ�ѧ����ʽ�� ��
�� B��C�ķ�Ӧ�У�B�����ڼ�����������ȥһ��ˮ���ӣ�����C��C������ֻ��1��̼ԭ��������ԭ�ӡ�C�Ľṹ��ʽ�� ��
�� P�Ľṹ��ʽ�� ��
�� ��������������B��ͬ���칹�干�У������֣� �֡�
a��������������ˮ��ΪM��N b��һ��������M����ת��ΪN
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ��������ƽ��ɽ���������ε��п��ԣ����ۣ���ѧ���� ���ͣ������
�߾���Z��һ����Ҫ�ĵ��ӱ�Ĥ���ϣ�P��һ�ֹ㷺Ӧ�õ����ܼ���������ij�о�С����Ƶ����ǵĺϳ�·�ߡ�

��֪������Ϣ��
��l molF��O2�г��ȼ�գ�����7��5 rriol O2������8 nl01 C02��3 mol H20��l mol F������NaHCO3��Һ��Ӧ����2 rriol C02����˴Ź�����������3�����շ塣
����֪�� ��R��R��������������ԭ�ӣ���
��R��R��������������ԭ�ӣ���
��A��һ��ȩ��B��C�ķ�Ӧ�У�B�����ڼ�����������ȥһ��ˮ���ӣ�����C��C������ֻ��1��̼ԭ��������ԭ�ӡ�
��D��������8��̼ԭ�ӣ�����������6��̼ԭ�ӣ��ҷ�����ֻ����һ����CH3��
��ش��������⣺
��1��X�Ľṹ��ʽ�� ��F�����к��еĺ��������ŵ������� ��
��2��д����Ӧ�ٵĻ�ѧ����ʽ ����Ӧ�ڵĻ�ѧ����ʽ�� ��
��3����Ӧ�۵Ļ�ѧ����ʽ�� ��
��4��C�Ľṹ��ʽ�� _��P�Ľṹ��ʽ��-��
��5����������������B��ͬ���칹�干�У������֣� �֡�
a��������������ˮ��ΪM��N b��-��������M����ת��ΪN
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A��ij������Һ��ֻ����Na+.CH3COO-.H+.OH-,�����Һ�м���������ˮ��c(CH3COO-)һ������c(Na+)�� c(NH4+)֮�͡�
B�������½�0.01molCH3COONa��0.004molHCl����ˮ�����Ƴ�0.5L�����Һ����Һ��n(CH3COO-)+ n(OH-)-n(H+)=0.006mol
C������������Һ�еμ�ϡ����õ���pH��5�Ļ����Һ��c(Na��)<c(NO3��)
D�����ʵ���Ũ����ȵ�NaF��NaCN��Һ���������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com