
| ���� | NaCl | KCl | CaO |
| ������/��kJ?mol-1�� | 786 | 715 | 3 401 |

| 6+2 |
| 2 |
| 6+2-2��4 |
| 2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
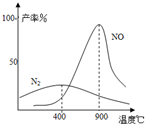 ��2009?��ɽģ�⣩��ҵ�ϳɰ����Ʊ�����һ��������������������£�
��2009?��ɽģ�⣩��ҵ�ϳɰ����Ʊ�����һ��������������������£�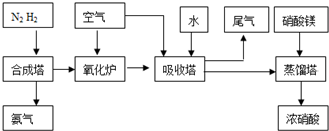
| ʱ�䣨h��Ũ�ȣ�mol/L�� | 0 | 1 | 2 | 3 | 4 |
| N2 | 1.500 | 1.400 | 1.200 | C1 | C1 |
| H2 | 4.500 | 4.200 | 3.600 | C2 | C2 |
| NH3 | 0 | 0.200 | 0.600 | C3 | C3 |
| 1 |
| a |
| 1 |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
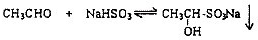
| �ʻ������� | CH3CHO | CH3COCH3 | C2H5COCH3 | CH3CH2CH2COCH3 |
| ���ʣ�1Сʱ�ڣ� | 88.7 | 56.2 | 36.4 | 23.4 |
| �ʻ������� | ��CH3��2CHCOCH3 | ��CH3��3CCOCH3 | C2H5COC2H5 | C6H5COCH3 |
| ���ʣ�1Сʱ�ڣ� | 12.3 | 5.6 | 2 | 1 |
| Cl2NaOH |
| NaOH |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com