
| |||||||||||
|
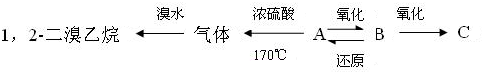
AµДПа¶Ф·ЦЧУЦКБї1.34g/LЎБ22.4Йэ/molЈЅ30 ФтBЎўCµДПа¶Ф·ЦЧУЦКБї¶јОЄ60 ЧојтКЅn(C)Јєn(H)Јєn(O) ЈЅ ЎаЧојтКЅОЄCH2O ЙиAµД·ЦЧУКЅОЄ(CH2O)n(CH2O)nЈЅ30 nЈЅ1A·ЦЧУКЅОЄCH2O BЎўC»ҐОЄН¬·ЦТмЅб№№Ј¬Йи·ЦЧУКЅОЄ(CH2O)n (CH2O)nЈЅ60nЈЅ2 BЎўCµД·ЦЧУКЅОЄC2H4O2 УЙМвТвЦЄAДЬ·ўЙъТшѕµ·ґУ¦ЛщТФAµДЅб№№КЅОЄ CПФЦРРФОЄјЧЛбјЧхҐЈ¬ЖдЅб№№КЅОЄ BПФЛбРФОЄТТЛбCH3COOH |


 ФД¶БїміµПµБРґр°ё
ФД¶БїміµПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
| Cu |
| Ўч |
| Cu |
| Ўч |
| ґЯ»ЇјБ |
| Ўч |
| ґЯ»ЇјБ |
| Ўч |
| Т»¶ЁМхјю |
| Т»¶ЁМхјю |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
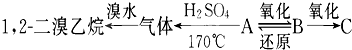
| Cu»тAg |
| Ўч |
| Cu»тAg |
| Ўч |
| Т»¶ЁМхјю |
| Т»¶ЁМхјю |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
| деЛ® |
| ЕЁБтЛб | ||
|
| Сх»Ї |
| »№Ф |
| Сх»Ї |
| Cu |
| Ўч |
| Cu |
| Ўч |
| ЕЁH2SO4 |
| Ўч |
| ЕЁH2SO4 |
| Ўч |
| Ўч |
| Ўч |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2013ЅмЙЅ¶«јГДПКАјНУў»ЄКµСйѧУёЯ¶юПВС§ЖЪЖЪЦРїј»ЇС§ѕнЈЁЅвОц°жЈ© МвРНЈєМоїХМв
УРAЎўBЎўCИэЦЦМюµДСЬЙъОпЈ¬П໥ת»Ї№ШПµИзПВЈє

ЖдЦРBїЙ·ўЙъТшѕµ·ґУ¦Ј¬CёъКЇ»ТКЇ·ґУ¦ІъЙъДЬК№іОЗеКЇ»ТЛ®±д»лЧЗµДЖшМеЎЈ
1.AЎўBЎўCµДЅб№№јтКЅєНГыіЖТАґОКЗ_______________________________Ўў_____________________________Ўў_______________________________ЎЈ
2.РґіцПВБР·ґУ¦µД»ЇС§·ЅіМКЅ AЎъB_______________________________________
BЎъC ________________________________________
BЎъA ___________________________________________
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2012ЅмєУДПКЎі¤ёрКРёЯ¶юПВС§ЖЪЖЪЦРїјКФ»ЇС§КФѕн МвРНЈєМоїХМв
ЈЁ12·ЦЈ©УРAЎўBЎўCИэЦЦМюµДСЬЙъОпЈ¬П໥ת»Ї№ШПµИзПВЈє
 Ј¬
Ј¬
ЖдЦРBїЙ·ўЙъТшѕµ·ґУ¦Ј¬CёъКЇ»ТКЇ·ґУ¦ІъЙъДЬК№КЇ»ТЛ®±д»лЧЗµДЖшМеЎЈФт
ЈЁ1Ј©НЖ¶ПAЎўBЎўCµДГыіЖТАґОКЗ___ _____Ўў___ _______Ўў___ _____
ЈЁ2Ј©РґіцПВБР·ґУ¦µД»ЇС§·ЅіМКЅ
AЎъB ____________________________________ ____________
BЎъC _________________________________ _________ __________
BЎъA __________________________________ _________
BµДТшѕµ·ґУ¦_________________________________ _______
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com