
�������ǵ䡱�ڼ䣬�������ᣨ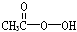 ���ǹ�Ϊʹ�õ���������������H2O2�ͱ����ᷴӦ��ȡ�������ڹ��������г����в�����H2O2���ⶨ��Ʒ�й�������Ũ��c0���漰���з�Ӧ��
���ǹ�Ϊʹ�õ���������������H2O2�ͱ����ᷴӦ��ȡ�������ڹ��������г����в�����H2O2���ⶨ��Ʒ�й�������Ũ��c0���漰���з�Ӧ��
�� ��MnO4����H2O2����H+ �T��Mn2+����O2ʮ��H2O
�� H2O2��2 ��2H+ �T I2��2H2O
��2H+ �T I2��2H2O
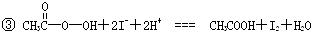
�� I2+2S2O32-�TS4O62-+2I-
��ش��������⣺
��l����ƽ��Ӧ�ٵ����ӷ���ʽ����ƽϵ���������·����ڣ���
��MnO-4����H2O2����H+ �T ��Mn2+����O2ʮ��H2O
��2����Na2S2O3����Һ�ζ�I2ʱ����Ӧ�ܣ�ѡ�õ�ָʾ����_____________________��
��3��ȡb0 mL����Һ��������ʹ��Һ�ữ������Ũ��Ϊa1 mol�� ��KMnO4����Һ�ζ����е�H2O2�����õ�KMnO4���Ϊb1 mL����Ӧ�٣��ζ�������KmnO4����������ᷴӦ������ȡb0 mL����Һ�����������KI����������ʹ��Һ�ữ����ʱ��������Ͳ�����H2O2���ܸ� KI��Ӧ���� I2����Ӧ�ںۣ͢�������Ũ��Ϊa2 mol��
��KMnO4����Һ�ζ����е�H2O2�����õ�KMnO4���Ϊb1 mL����Ӧ�٣��ζ�������KmnO4����������ᷴӦ������ȡb0 mL����Һ�����������KI����������ʹ��Һ�ữ����ʱ��������Ͳ�����H2O2���ܸ� KI��Ӧ���� I2����Ӧ�ںۣ͢�������Ũ��Ϊa2 mol�� ��Na2S2O3����Һ�ζ����ɵ�I2������Na2S2O3��Һ���Ϊb2 mL��
��Na2S2O3����Һ�ζ����ɵ�I2������Na2S2O3��Һ���Ϊb2 mL��
���������ʵ�����ݼ�����������Ũ�ȣ��ú�a1��a2��b0��b1��b2�Ĵ���ʽ��ʾ����
c0�� ________________________��
��4��Ϊ�������Һ�й��������Ũ��c0�������KI�������ѹ�����û��ȷ�������Ƿ�Ӱ��ⶨ��� _______________�����ǻ��
��1��2MnO4-��5H2O2��6H+��2Mn2+��5O2ʮ8H2O
��2��������Һ ��3�� ��4����
��4����
��������
�����������1�����ݷ���ʽ��֪��MnԪ�صĻ��ϼ۴ӣ�7�۽ϵ͵���2�ۣ��õ�5�����ӡ�˫��ˮ����Ԫ�صĻ��ϼ۴ӣ�1�����ߵ�0�ۣ�ʧȥ1�����ӣ����Ը��ݵ��ӵĵ�ʧ�غ��֪���������뻹ԭ�������ʵ���֮����2:5������ƽ��ķ���ʽ��2MnO4-��5H2O2��6H+��2Mn2+��5O2ʮ8H2O��
��2�����ڵ�����������ɫ��������Na2S2O3����Һ�ζ�I2ʱ����Ӧ�ܣ�ѡ�õ�ָʾ���ǵ�����Һ��
��3�����ݷ�Ӧ�ٿ�֪����Һ��˫��ˮ�����ʵ�����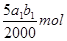 ������ݷ�Ӧ�ڿ�֪��˫��ˮ�͵⻯�ط�Ӧ���ɵĵ��ʵ�����ʵ�����
������ݷ�Ӧ�ڿ�֪��˫��ˮ�͵⻯�ط�Ӧ���ɵĵ��ʵ�����ʵ�����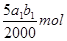 �����ݷ�Ӧ�ܿ�֪����Ӧ�����ɵĵ��ʵ�����ʵ���������
�����ݷ�Ӧ�ܿ�֪����Ӧ�����ɵĵ��ʵ�����ʵ���������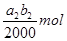 �����Թ���������⻯�ط�Ӧ���ɵĵ��ʵ�����ʵ�����
�����Թ���������⻯�ط�Ӧ���ɵĵ��ʵ�����ʵ����� �����Ը��ݢ۷�Ӧ��֪��������������ʵ�����
�����Ը��ݢ۷�Ӧ��֪��������������ʵ����� �����Թ��������Ũ����
�����Թ��������Ũ���� mol/L��
mol/L��
��4�����ݣ�3���еķ�����֪���ⶨ�����⻯�ص�����û�й�ϵ�����Բ���Ӱ������
���㣺����������ԭ��Ӧ����ʽ����ƽ��ָʾ����ѡ������Ũ�ȵIJⶨ
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ����������������ⷽ����ָ����ѵ��������������ѧ�������������������ѧ���淶���Ͻ���ʵ�����������������Ҫ����ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| a2b2-5a1b1 |
| 2b0 |
| a2b2-5a1b1 |
| 2b0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�043
���������ǵ䡱�ڼ䣬��������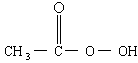 �ǹ�Ϊʹ�õ���������������
�ǹ�Ϊʹ�õ���������������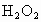 �ͱ����ᷴӦ��ȡ�������ڹ��������г����в�����
�ͱ����ᷴӦ��ȡ�������ڹ��������г����в�����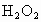 ���ⶨ��Ʒ�й�������Ũ��
���ⶨ��Ʒ�й�������Ũ��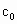 ���漰���з�Ӧ��
���漰���з�Ӧ��
��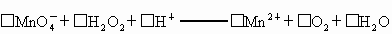
��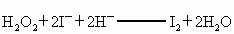
��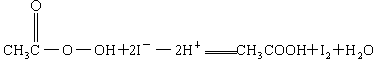
��
��ش��������⣺
(1)��ƽ��Ӧ�ٵ����ӷ���ʽ(�����ѧ�������������·�����)��

(2)��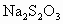 ����Һ�ζ�
����Һ�ζ�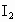 ʱ(��Ӧ��)ѡ�õ�ָʾ����__________��
ʱ(��Ӧ��)ѡ�õ�ָʾ����__________��
(3)ȡ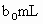 ����Һ��������ʹ��Һ�ữ������Ũ��Ϊ
����Һ��������ʹ��Һ�ữ������Ũ��Ϊ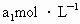 ����Һ�ζ����е�
����Һ�ζ����е�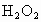 �����õ�
�����õ� ���Ϊ
���Ϊ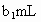 (��Ӧ�٣��ζ�������
(��Ӧ�٣��ζ������� ����������ᷴӦ)��
����������ᷴӦ)��
��ȡ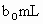 ����Һ�����������KI����������ʹ��Һ���ữ����ʱ��������Ͳ�����
����Һ�����������KI����������ʹ��Һ���ữ����ʱ��������Ͳ�����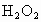 ���ܸ�KI��Ӧ����
���ܸ�KI��Ӧ����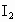 (��Ӧ�ں͢�)������Ũ��Ϊ
(��Ӧ�ں͢�)������Ũ��Ϊ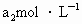 ��
��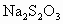 ����Һ�ζ����ɵ�
����Һ�ζ����ɵ�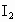 ������
������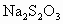 ��Һ���Ϊ
��Һ���Ϊ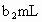 ��
��
���������ʵ�����ݼ�����������Ũ��(�ú�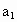 ��
�� ��
��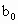 ��
��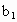 ��
��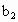 �Ĵ���ʽ��ʾ)��
�Ĵ���ʽ��ʾ)��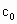 =__________��
=__________��
(4)Ϊ�������Һ�й��������Ũ��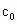 �������KI�������ѹ�������û��ȷ�������Ƿ�Ӱ��ⶨ���______(��ǡ���)��
�������KI�������ѹ�������û��ȷ�������Ƿ�Ӱ��ⶨ���______(��ǡ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��_____![]() +_____H2O2+______H+����_____Mn2++______O2+______H2O
+_____H2O2+______H+����_____Mn2++______O2+______H2O
��H2O2+2I-+2H+====I2+2H2O
��CH3COOOH+2I-+2H+====CH3COOH+I2+H2O
��I2+2![]() ====S4O62-+2I-
====S4O62-+2I-
��ش��������⣺
(1)��ƽ��Ӧ�ٵ����ӷ���ʽ(��ƽ��ѧ����������������������ֱ����)��
![]() +_____H2O2+_____H+����______Mn2++_____O2+_____H2O
+_____H2O2+_____H+����______Mn2++_____O2+_____H2O
(2)��Na2S2O3����Һ�ζ�I2ʱ(��Ӧ��)ѡ�õ�ָʾ����________��
(3)ȡb0 mL����Һ��������ʹ��Һ�ữ������Ũ��Ϊa1 mol��L-1��KMnO4����Һ�ζ����е�H2O2�����õ�KMnO4���Ϊb1 mL(��Ӧ�٣��ζ������У�KMnO4����������ᷴӦ)��
��ȡb0 mL����Һ�����������KI����������ʹ��Һ�ữ����ʱ��������Ͳ�����H2O2���ܸ�KI��Ӧ����I2(��Ӧ�ں͢�)������Ũ��Ϊa2 mol��L-1��Na2S2O3����Һ�ζ����ɵ�I2������Na2S2O3��Һ���Ϊb2 mL��
���������ʵ�����ݼ�����������Ũ�ȡ�
c0=________(�ú�a1��a2��b0��b1��b2�Ĵ���ʽ��ʾ)��
(4)Ϊ�������Һ�й��������Ũ��c0�������KI�������ѹ�����û��ȷ�������Ƿ�Ӱ��ⶨ���______(��ǡ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ̫�����и�����ѧ����ĩǰ�¿���ѧ�Ծ����������� ���ͣ������
�������ǵ䡱�ڼ䣬�������ᣨ ���ǹ�Ϊʹ�õ���������������H2O2�ͱ����ᷴӦ��ȡ�������ڹ��������г����в�����H2O2���ⶨ��Ʒ�й�������Ũ��c0���漰���з�Ӧ��
���ǹ�Ϊʹ�õ���������������H2O2�ͱ����ᷴӦ��ȡ�������ڹ��������г����в�����H2O2���ⶨ��Ʒ�й�������Ũ��c0���漰���з�Ӧ��
�� ��MnO4����H2O2����H+ �T��Mn2+����O2ʮ��H2O
�� H2O2��2 ��2H+ �T I2��2H2O
��2H+ �T I2��2H2O
�� I2+2S2O32-�TS4O62-+2I-
��ش��������⣺
��l����ƽ��Ӧ�ٵ����ӷ���ʽ����ƽϵ���������·����ڣ���
��MnO-4����H2O2����H+ �T ��Mn2+����O2ʮ��H2O
��2����Na2S2O3����Һ�ζ�I2ʱ����Ӧ�ܣ�ѡ�õ�ָʾ����_____________________��
��3��ȡb0 mL����Һ��������ʹ��Һ�ữ������Ũ��Ϊa1 mol�� ��KMnO4����Һ�ζ����е�H2O2�����õ�KMnO4���Ϊb1 mL����Ӧ�٣��ζ�������KmnO4����������ᷴӦ������ȡb0 mL����Һ�����������KI����������ʹ��Һ�ữ����ʱ��������Ͳ�����H2O2���ܸ� KI��Ӧ���� I2����Ӧ�ںۣ͢�������Ũ��Ϊa2 mol��
��KMnO4����Һ�ζ����е�H2O2�����õ�KMnO4���Ϊb1 mL����Ӧ�٣��ζ�������KmnO4����������ᷴӦ������ȡb0 mL����Һ�����������KI����������ʹ��Һ�ữ����ʱ��������Ͳ�����H2O2���ܸ� KI��Ӧ���� I2����Ӧ�ںۣ͢�������Ũ��Ϊa2 mol�� ��Na2S2O3����Һ�ζ����ɵ�I2������Na2S2O3��Һ���Ϊb2 mL��
��Na2S2O3����Һ�ζ����ɵ�I2������Na2S2O3��Һ���Ϊb2 mL��
���������ʵ�����ݼ�����������Ũ�ȣ��ú�a1��a2��b0��b1��b2�Ĵ���ʽ��ʾ����
c0�� ________________________��
��4��Ϊ�������Һ�й��������Ũ��c0�������KI�������ѹ�����û��ȷ�������Ƿ�Ӱ��ⶨ��� _______________�����ǻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡ������ѧ�ڵ�һ��ģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��8�֣��������ǵ䡱�ڼ䣬�������ᣨ 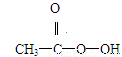 ���ǹ�Ϊʹ�õ���������������H2O2�ͱ����ᷴӦ��ȡ�������ڹ��������г����в�����H2O2���ⶨ��Ʒ�й�������Ũ��c0���漰���з�Ӧ��
���ǹ�Ϊʹ�õ���������������H2O2�ͱ����ᷴӦ��ȡ�������ڹ��������г����в�����H2O2���ⶨ��Ʒ�й�������Ũ��c0���漰���з�Ӧ��
�� ��MnO4-����H2O2����H+ = ��Mn2+����O2ʮ��H2O
��
H2O2��2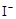 ��2H+ = I2��2H2O
��2H+ = I2��2H2O
��
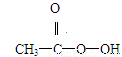 ��2
��2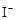 ��2H+ = CH3COOH��I2��H2O
��2H+ = CH3COOH��I2��H2O
�� I2��2S2O32- = 2I-��S4O62-
��ش��������⣺
��l����ƽ��Ӧ�ٵ����ӷ���ʽ����ƽϵ���������·����ڣ���
MnO4-�� H2O2�� H+ === Mn2+�� O2ʮ H2O
��2����Na2S2O3����Һ�ζ�I2ʱ����Ӧ�ܣ�ѡ�õ�ָʾ����________________________
��3��ȡb0 mL����Һ��������ʹ��Һ�ữ������Ũ��Ϊa1 mol��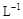 ��KMnO4����Һ�ζ����е�H2O2�����õ�KMnO4���Ϊb1 mL����Ӧ�٣��ζ�������KMnO4����������ᷴӦ����
��KMnO4����Һ�ζ����е�H2O2�����õ�KMnO4���Ϊb1 mL����Ӧ�٣��ζ�������KMnO4����������ᷴӦ����
��ȡb0 mL����Һ�����������KI����������ʹ��Һ�ữ����ʱ��������Ͳ�����H2O2���ܸ� KI��Ӧ���� I2����Ӧ�ںۣ͢�������Ũ��Ϊa2
mol��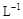 ��Na2S2O3����Һ�ζ����ɵ�I2������Na2S2O3��Һ���Ϊb2 mL��
��Na2S2O3����Һ�ζ����ɵ�I2������Na2S2O3��Һ���Ϊb2 mL��
���������ʵ�����ݼ�����������Ũ�ȣ��ú�a1��a2��b0��b1��b2�Ĵ���ʽ��ʾ����
c0�� ___________ ��
(4��Ϊ�������Һ�й��������Ũ��c0�������KI�������ѹ�����û��ȷ�������Ƿ�Ӱ��ⶨ��� _______________�����ǻ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com