
| A�� | ��pH=2������Һϡ�͵�ԭ����$\frac{1}{10}$����Һ��pHһ����Ϊ3 | |
| B�� | ��pH=2�������pH=4������������Ϻ���Һ��pH��Ϊ3 | |
| C�� | ��pH=12��Ba��OH��2��Һϡ�͵�ԭ����$\frac{1}{10}$����Һ��pH��Ϊ13 | |
| D�� | ��0.005mol•L-1��������pH=12��Ba��OH��2��Һ�������Ϻ���Һ��pHΪ7 |
���� A�������ˮϡ�ͻ�ٽ����룬�����ӵ����ʵ�������
B������Һ��Ϻ��Һ��pH��������Һ��pH��ƽ��ֵ����Ҫ����c=$\frac{n}{V}$���㣻
C������Һϡ�ͺ���Һ��pH��С��ϡ�ͺ����Һ��pHӦ��Ϊ11��
D�����������������Һ�������ϣ����������Ӻ�����������Ũ�ȼ��㣮
��� �⣺A����pH=2������Һϡ��10������Ϊǿ�ᣬ����Һ��pH��Ϊ3����Ϊ������ʣ�ϡ�ͺ�����ĵ���̶�������Һ�������ӵ����ʵ���������Һ��pHС��3����A����
B��pH=2��������Һ��������Ũ��Ϊ0.01mol/L��pH=4��������Һ��������Ũ��Ϊ0.0001mol/L������Һ�������Ϻ���Һ��������Ũ��Ϊ��$\frac{0.01+0.0001}{2}$mol/L��0.001�����Ի��Һ��pHһ������3����B����
C��pH=12��Ba��OH��2��Һ������������Ũ��Ϊ0.01mol/L��ϡ��10��������������Ũ��Ϊ0.001mol/L��ϡ�ͺ���Һ��pH��Ϊ11����C����
D��0.005mol•L-1��������������Ũ��Ϊ0.01mol/L��pH=12������������Һ������������Ũ��Ϊ��0.01mol/L������Һ�������Ϻ�ǡ����ȫ��Ӧ��������Һ�����ԣ���pH=7����D��ȷ��
��ѡD��
���� ���⿼����pH�ļ��㡢����ϵĶ����жϣ���Ŀ�Ѷ��еȣ�ע���й���ҺpH�ļ��㷽������ȷ��Һ���������ҺpH�Ĺ�ϵ��ѡ��BΪ�״��㣬ע�������Һ��pHʱ����ֱ����pHֵ���㣬��Ҫ��������Һ��������Ũ�ȣ�Ȼ�����pH����ʽ���㣮


 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
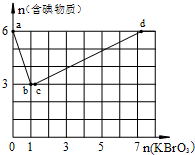 ����6mol KI��������Һ����μ���KBrO3��Һ�����������к������ʵ����ʵ�����������KBrO3�����ʵ����Ĺ�ϵ��ͼ��ʾ��
����6mol KI��������Һ����μ���KBrO3��Һ�����������к������ʵ����ʵ�����������KBrO3�����ʵ����Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����� | B�� | ������ | C�� | �������� | D�� | ����ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ȵ��¶Ȳ�ͬ | B�� | �õ��IJ�Ʒ��ͬ | ||
| C�� | ǰ��Ҫ�������������߲��� | D�� | �����ǻ�ѧ�仯�������������仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѹ | B�� | ��ѹ | C�� | ��СB��Ũ�� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��NO3-��Al3+��Cl- | B�� | Na+��Al3+��S2-��NO3- | ||
| C�� | MnO4-��K+��SO42-��Na+ | D�� | K+��SO42-��HCO3-��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
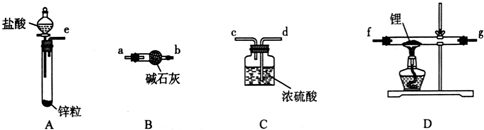
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10mL��������Ϊ98%��H2SO4����10mLˮϡ�ͺ�H2SO4��������������49% | |
| B�� | ����0.1mol/L��Na2CO3��Һ480mL������500mL����ƿ | |
| C�� | �ڱ���£���22.4L��������1Lˮ�У��õ�1mol/L�İ�ˮ | |
| D�� | ͬ��ͬѹ�£�20mLCH4��60mLO2������ԭ����֮��Ϊ5��6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com