
ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēÓŅĶ¼ĖłŹ¾£ŗ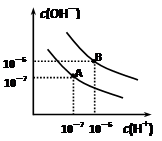
¢ÅŌņ25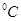 Ź±Ė®µÄµēĄėĘ½ŗāĒśĻßÓ¦ĪŖ £ØĢī”°A”±»ņ”°B”±£©”£
Ź±Ė®µÄµēĄėĘ½ŗāĒśĻßÓ¦ĪŖ £ØĢī”°A”±»ņ”°B”±£©”£
¢Ę25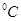 Ź±£¬½«
Ź±£¬½«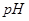 £½8µÄNaOHČÜŅŗÓė
£½8µÄNaOHČÜŅŗÓė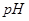 £½5µÄ
£½5µÄ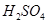 ČÜŅŗ»ģŗĻ£¬ČōĖłµĆ»ģŗĻČÜŅŗµÄ
ČÜŅŗ»ģŗĻ£¬ČōĖłµĆ»ģŗĻČÜŅŗµÄ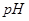 £½7£¬ŌņNaOHČÜŅŗÓė
£½7£¬ŌņNaOHČÜŅŗÓė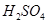 ČÜŅŗµÄĢå»ż±ČĪŖ ”£
ČÜŅŗµÄĢå»ż±ČĪŖ ”£
¢Ē95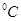 Ź±£¬0.1 mol/LµÄNaOHČÜŅŗµÄpHÖµŹĒ ”£
Ź±£¬0.1 mol/LµÄNaOHČÜŅŗµÄpHÖµŹĒ ”£
¢Č95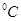 Ź±£¬Čō100Ģå»ż
Ź±£¬Čō100Ģå»ż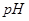 1£½
1£½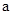 µÄijĒæĖįČÜŅŗÓė1Ģå»ż
µÄijĒæĖįČÜŅŗÓė1Ģå»ż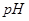 2£½bµÄijĒæ¼īČÜŅŗ»ģŗĻŗóČÜŅŗ³ŹÖŠŠŌ£¬Ōņ»ģŗĻĒ°£¬
2£½bµÄijĒæ¼īČÜŅŗ»ģŗĻŗóČÜŅŗ³ŹÖŠŠŌ£¬Ōņ»ģŗĻĒ°£¬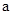 ÓėbÖ®¼äÓ¦Āś×ćµÄ¹ŲĻµŹĒ
ÓėbÖ®¼äÓ¦Āś×ćµÄ¹ŲĻµŹĒ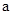 = £ØÓĆŗ¬bµÄ“śŹżŹ½±ķŹ¾£©,a+b_______14£ØĢī”°<”±”¢”°=”±»ņ”°>”±£©”£
= £ØÓĆŗ¬bµÄ“śŹżŹ½±ķŹ¾£©,a+b_______14£ØĢī”°<”±”¢”°=”±»ņ”°>”±£©”£
¢ÅA ¢Ę10:1 ¢Ē11 ¢Č14£b£¬£½
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©µēĄėŹĒĪüČČ£¬ŌņÉżøßĪĀ¶Č£¬µēĄė³Ģ¶ČŌö¼Ó£¬Ė®ÖŠĒāĄė×ÓÅضČŌö¼Ó£¬ĖłŅŌ25 ”ꏱĖ®µÄµēĄėĘ½ŗāĒśĻßÓ¦ĪŖA”£
£Ø2£©ČōĖłµĆ»ģŗĻČÜŅŗµÄpH=7£¬Ōņ £¬½āµĆ
£¬½āµĆ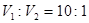 ”£
ӣ
£Ø3£©95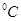 Ź±£¬0.1 mol/LµÄNaOHČÜŅŗÖŠOH£µÄÅØ¶ČŹĒ0.1mol/L£¬ŌņČÜŅŗÖŠĒāĄė×ÓµÄÅØ¶ČŹĒ10£11mol/L£¬ŌņČÜŅŗµÄpHÖµŹĒ11”£
Ź±£¬0.1 mol/LµÄNaOHČÜŅŗÖŠOH£µÄÅØ¶ČŹĒ0.1mol/L£¬ŌņČÜŅŗÖŠĒāĄė×ÓµÄÅØ¶ČŹĒ10£11mol/L£¬ŌņČÜŅŗµÄpHÖµŹĒ11”£
£Ø4£©95”ꏱČō100Ģå»żpH1=aµÄijĒæĖįČÜŅŗÓė1Ģå»żpH2=bµÄijĒæ¼īČÜŅŗ»ģŗĻŗóČÜŅŗ³ŹÖŠŠŌ£¬Ōņ £¬ĖłŅŌøĆĒæĖįµÄpH1ÓėĒæ¼īµÄpH2Ö®¼äÓ¦Āś×ćµÄ¹ŲĻµŹĒa+b=14”£
£¬ĖłŅŌøĆĒæĖįµÄpH1ÓėĒæ¼īµÄpH2Ö®¼äÓ¦Āś×ćµÄ¹ŲĻµŹĒa+b=14”£
æ¼µć£ŗæ¼²éĶā½ēĢõ¼ž¶ŌĖ®µÄµēĄėĘ½ŗāµÄÓ°Ļģ”¢pHµÄ¼ĘĖć
µćĘĄ£ŗøĆĢāŹĒøßæ¼ÖŠµÄ³£¼ūĢāŠĶ£¬ŹōÓŚÖŠµČÄѶȵďŌĢā”£ŹŌĢā×ŪŗĻŠŌĒ棬²ąÖŲ¶Ōѧɜ»ł“”ÖŖŹ¶µÄ¹®¹ĢÓėѵĮ·£¬ÓŠĄūÓŚÅąŃųѧɜµÄĀß¼ĶĘĄķÄÜĮ¦£¬ĢįøßѧɜĮé»īŌĖÓĆ»ł“”ÖŖŹ¶½ā¾öŹµ¼ŹĪŹĢāµÄÄÜĮ¦”£øĆĢāµÄ¹Ų¼üŹĒĆ÷Č·Ėę×ÅĪĀ¶ČµÄÉżøߣ¬Ė®µÄĄė×Ó»ż³£ŹżŹĒŌö“ó£¬ŌŚ¼ĘĖćpHŹ±ŠčŅŖĮé»īŌĖÓĆ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ
ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø1£©Š“³öĻĀĮŠĪļÖŹČÜÓŚĖ®µÄµēĄė·½³ĢŹ½¢Ł±ł“×ĖįČÜÓŚĖ®£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹČÜÓŚĖ®µÄµēĄė·½³ĢŹ½¢Ł±ł“×ĖįČÜÓŚĖ®£ŗ| 3 |
| 2 |
| 3 |
| 2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ
ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
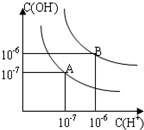 ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ
ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
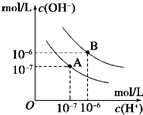 ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·Ø“ķĪóµÄ£Ø””””£©
ŅŃÖŖĖ®ŌŚ25”ęŗĶ95”ꏱ£¬ĘäµēĄėĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·Ø“ķĪóµÄ£Ø””””£©| A”¢AĒśĻß“ś±ķ25”ꏱĖ®µÄµēĄėĘ½ŗāĒśĻß | B”¢µ±95”ꏱ£¬pH=6µÄČÜŅŗ³ŹÖŠŠŌ | C”¢25”ꏱ£¬½«10mLpH=12µÄNaOHČÜŅŗÓė1mLpH=1µÄH2SO4ČÜŅŗ»ģŗĻ£¬ĖłµĆČÜŅŗµÄpH=7 | D”¢95”ꏱ£¬µČĢå»żµČĪļÖŹµÄĮæÅØ¶ČµÄHAČÜŅŗŗĶNaOHČÜŅŗ»ģŗĻŗ󣬵±»ģŗĻČÜŅŗµÄpH=6Ź±£¬ĖµĆ÷HAĖįĪŖČõĖį |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com