
 ������N2�����ʵ���Ϊ0.6mol����
������N2�����ʵ���Ϊ0.6mol���� __________
__________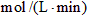 ��
��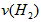 __________
__________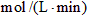 ��
��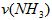 __________
__________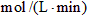 ��
�� ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2SO3��l����2min����O2�����ʵ���Ϊ1.6mol����
2SO3��l����2min����O2�����ʵ���Ϊ1.6mol�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2SO3��2minʱ�����O2�����ʵ���Ϊ1.6mol����
2SO3��2minʱ�����O2�����ʵ���Ϊ1.6mol���� 2SO3��g����H=-2.5QkJ?mol-1
2SO3��g����H=-2.5QkJ?mol-1 2SO3��g����H=-2.5QkJ?mol-1
2SO3��g����H=-2.5QkJ?mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��7�֣������Ϊ2L�������м���1mol N2��6mol H2���п��淴Ӧ��N2+3H2 2NH3��
![]() ����N2�����ʵ���Ϊ0.6mol����
����N2�����ʵ���Ϊ0.6mol����
��1��2min�ڣ�N2�����ʵ���������0.4mol��H2�����ʵ���������________mol��NH3��������������________mol��
��2������N2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����Ϊ mol/(L��min)��
��3������H2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����Ϊ mol/(L��min)��
��4������NH3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����Ϊ mol/(L��min)��
��5��ͨ���������㣬����ʲô���֣�____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�갲��ʡ����ũ����ѧ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��7�֣������Ϊ2L�������м���1mol N2��6mol H2���п��淴Ӧ��N2+3H2 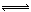 2NH3��
2NH3�� ����N2�����ʵ���Ϊ0.6mol����
����N2�����ʵ���Ϊ0.6mol����
��1��2min�ڣ�N2�����ʵ���������0.4mol��H2�����ʵ���������________mol��NH3��������������________mol��
��2������N2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����Ϊ mol/(L��min)��
��3������H2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����Ϊ mol/(L��min)��
��4������NH3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����Ϊ mol/(L��min)��
��5��ͨ���������㣬����ʲô���֣�____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��7�֣������Ϊ2L�������м���1mol N2��6mol H2���п��淴Ӧ��N2+3H2 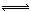 2NH3��
2NH3�� ����N2�����ʵ���Ϊ0.6mol����
����N2�����ʵ���Ϊ0.6mol����
��1��2min�ڣ�N2�����ʵ���������0.4mol��H2�����ʵ���������________mol��NH3��������������________mol��
��2������N2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����Ϊ mol/(L��min)��
��3������H2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����Ϊ mol/(L��min)��
��4������NH3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ����Ϊ mol/(L��min)��
��5��ͨ���������㣬����ʲô���֣�____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com