
 ����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��
����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��
| �������� | Fe��OH��3 | Al��OH��3 | Mg��OH��2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
ʵ�鲽����ͼ�ף�
��ش��������⣺
��1�������ٵ�����______
��2��������Һ�����Ƿ���Fe3+�ķ���ʽ �����ŷ�����______
��3������A��өʯ��CaF2���������ữ�¿����Ʊ�ʯ�࣬д����Ӧ����ʽ______
��4���ӳ��������B����ȡD��Ӧ����һ���Լ����з��룬�䷴Ӧ�����ӷ���ʽΪ______
��5����������Ӧ������ҺpH�ĺ�����Χ��______������ţ���
A����1.5B��1.5��3.3 ���� C��7��8�� ���� D������9.4
��6��Ϊ̽�����õ�ˮ�ϼ�ʽ̼��þ[xMgCO3?yMg��OH��2?zH2O]����ɣ�ȡ��18.2g g��װ��A�IJ������У��밴����-����˳������װ�����Ӻã���ͼ�ң�������ţ�װ�ÿ��ظ�ʹ�ã���______��װ��CӦʢ���Լ���______�� ��ַ�Ӧ�����ʵ����Ӳ�ʲ�������ʣ�����8.0g�����ų�6.6gCO2���壬��x��y��z=______��
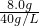 =0.2mol/L�����ų�6.6gCO2���壬������̼���ʵ���=
=0.2mol/L�����ų�6.6gCO2���壬������̼���ʵ���=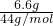 =0.15mol���ƶ�̼��þ���ʵ���Ϊ0.15mol������=0.15mol��84g/mol=12.6g��������þ���ʵ���Ϊ0.05mol������=0.05mol��58g/mol=2.9g���ᾧˮ������Ϊ18.2g-12.6g-2.9g=2.7g�����ʵ���=
=0.15mol���ƶ�̼��þ���ʵ���Ϊ0.15mol������=0.15mol��84g/mol=12.6g��������þ���ʵ���Ϊ0.05mol������=0.05mol��58g/mol=2.9g���ᾧˮ������Ϊ18.2g-12.6g-2.9g=2.7g�����ʵ���=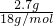 =0.15mol����x��y��z=0.15��0.05��0.15=3��1��3��
=0.15mol����x��y��z=0.15��0.05��0.15=3��1��3��

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���������� | �������↑ʼ����ʱ��pH | ����������ȫ������pH |
| Fe3+ | 1.9 | 3.2 |
| Mg2+ | 9.4 | 11.6 |
| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���������� | �������↑ʼ����ʱ��pH | ����������ȫ����ʱ��pH |
| Fe3+ | 1.9 | 3.2 |
| Mg2+ | 9.4 | 11.6 |
| ||
| ���� |
| ||
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��
����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��| �������� | Fe��OH��3 | Al��OH��3 | Mg��OH��2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��17�֣�����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɡ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
ʵ�鲽�����£�
��ش��������⣺
(1)ʵ������ɲ��������õ��IJ���������________________ ��
(2)������Һ�����Ƿ���Fe3���IJ����������___________________________
_____________________________________________________________________��
(3)�ӳ��������B����ȡD��Ӧ����һ���Լ����з��룬�䷴Ӧ�����ӷ���ʽΪ________________________________________________________________________��
�ٽ���__________�� �� (������дʵ���������)��
(4)��������Ӧ������ҺpH�ĺ�����Χ��____(�����)��
A����1.5 B��1.5��3.3
C��7��8 D������9.4
(5)Ϊ̽�����õ�ˮ�ϼ�ʽ̼��þ[xMgCO3��yMg(OH)2��zH2O]����ɣ�ȡ��7.28 g��װ��A�IJ������У��밴����D����˳������װ�����Ӻ�(����ţ�װ�ÿ��ظ�ʹ��)��________________________________________________________________________��
װ��CӦʢ���Լ���______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ����ѧУ������ѧ�����ۺϻ�ѧ�Ծ� ���ͣ�ʵ����
��17�֣�����ʯ���Կ�����MgO��Fe2O3��Al2O3��SiO2��ɡ�ʵ����������ʯΪԭ����ȡˮ�ϼ�ʽ̼��þ����֪��
|
�������� |
Fe(OH)3 |
Al(OH)3 |
Mg(OH)2 |
|
��ʼ����pH |
1.5 |
3.3 |
9.4 |
ʵ�鲽�����£�

��ش��������⣺
(1)ʵ������ɲ��������õ��IJ���������________________ ��
(2)������Һ�����Ƿ���Fe3���IJ����������___________________________
_____________________________________________________________________��
(3)�ӳ��������B����ȡD��Ӧ����һ���Լ����з��룬�䷴Ӧ�����ӷ���ʽΪ________________________________________________________________________��
�ٽ���__________�� �� (������дʵ���������)��
(4)��������Ӧ������ҺpH�ĺ�����Χ��____(�����)��
A����1.5 B��1.5��3.3
C��7��8 D������9.4
(5)Ϊ̽�����õ�ˮ�ϼ�ʽ̼��þ[xMgCO3��yMg(OH)2��zH2O]����ɣ�ȡ��7.28 g��װ��A�IJ������У��밴����D����˳������װ�����Ӻ�(����ţ�װ�ÿ��ظ�ʹ��)��________________________________________________________________________��
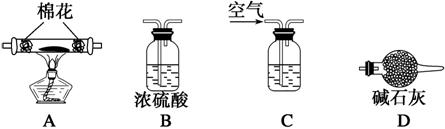
װ��CӦʢ���Լ���______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com