ПМЕуЃКЛЏбЇЗНГЬЪНЕФгаЙиМЦЫу,гУИЧЫЙЖЈТЩНјаагаЙиЗДгІШШЕФМЦЫу
зЈЬтЃК
ЗжЮіЃКЃЈ1ЃЉЕШжЪСПЕФШ§жжЮяжЪЃКЂйДПОЛЕФNa
2CO
3 a gЃЛЂкNa
2CO
3гыNaHCO
3ЕФЛьКЯЮяa gЃЛЂлДПОЛЕФNaHCO
3 a gЃЌЗжБ№ЫуГіЫќУЧЮяжЪЕФСПЃЌ
гыбЮЫсЭъШЋЗДгІЪБЃЌЗжБ№СаГіNa
2CO
3ЁЂNaHCO
3гыбЮЫсЗДгІЕФБШР§ЙиЯЕЪБЃЌЧѓГіHClЕФСПЃЎ
гыбЮЫсЭъШЋЗДгІЪБЃЌЗжБ№СаГіNa
2CO
3ЁЂNaHCO
3гыЩњГЩCO
2ЕФБШР§ЙиЯЕЪБЃЌЧѓГіCO
2ЕФСП
ЃЈ2ЃЉНЋag Na
2CO
3КЭNaHCO
3ЕФЛьКЯЮяГфЗжМгШШЃЌNaHCO
3ЕФЗжНтЗНГЬЪНЃЌОнВюСПЗЈНтЬтЃЛ
ЃЈ3ЃЉИљОнЬтвтжЊЃЌдкЗДгІжаPbO
2ЕУЕчзгзЊЛЏЮЊЮЊPbЃЈNO
3ЃЉ
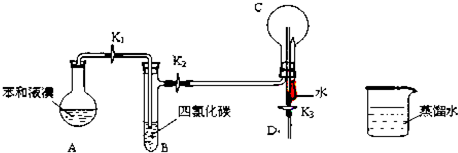
2ЃЌЫљвдЖўбѕЛЏЧІЪЧбѕЛЏМСЃЌдђЖўМлУЬЪЇЕчзгзїЛЙдМСЃЌШмвКГЪКьЩЋЃЌЫЕУїЖўМлУЬРызгБЛбѕЛЏЩњГЩИпУЬЫсИљРызгЃЌИљОнЗДгІЮяКЭЩњГЩЮяаДГіЯргІЕФРызгЗНГЬЪНЃЛ
ЃЈ4ЃЉвРОнШШЛЏбЇЗНГЬЪНЕФЪщаДЗНЗЈЃЌБъзЂЮяжЪОлМЏзДЬЌКЭЖдгІьЪБфаДГіЃЌвРОнИЧЫЙЖЈТЩМЦЫуЗДгІЕФьЪБфЃЛ
ЃЈ5ЃЉ2.24LЃЈБъПіЯТЃЉCO
2ЦјЬхЕФЮяжЪЕФСПЮЊ0.1molЃЌЖўбѕЛЏЬМЭъШЋЗДгІЃЌ
ШєжЛЗЂЩњЃКCO
2+2KOH=K
2CO
3+H
2OЃЌдђЩњГЩ0.1molЕФK
2CO
3ЃЌЦфжЪСП=0.1molЁС138g/mol=13.8gЃЛ
ШєжЛЗЂЩњЃКCO
2+KOH=KHCO
3ЃЌдђЩњГЩ0.1molЕФKHCO
3ЃЌЦфжЪСП=0.1molЁС100g/mol=10gЃЌ
гЩгк13.8gЃО11.9gЃО10.0gЃЌЫљвдЕУЕНЕФАзЩЋЙЬЬхЪЧK
2CO
3КЭKHCO
3ЕФЛьКЯЮяЃЌ
ЩшАзЩЋЙЬЬхжаK
2CO
3 x molЃЌKHCO
3 y molЃЌИљОнCдЊЫиЪиКуМАЖўепжЪСПСаЗНГЬМЦЫуЃЌдйИљОнМиРызгЪиКуМЦЫуKOHЮяжЪЕФСПЃЌИљОнc=
МЦЫуKOHШмвКЮяжЪЕФСПХЈЖШЃЎ
НтД№ЃК
НтЃКЃЈ1ЃЉЕШжЪСПЕФШ§жжЮяжЪЃКЂйДПОЛЕФNa
2CO
3 a gЃЌЮяжЪЕФСПЮЊ
molЃЛЂкNa
2CO
3гыNaHCO
3ЕФЛьКЯЮяa gЃЌЮяжЪЕФСПЮЊ
molЁЋ
molжЎМфЃЛ
ЂлДПОЛЕФNaHCO
3 a gЃЌЮяжЪЕФСПЮЊ
mol
AЃЎгыбЮЫсЭъШЋЗДгІЪБЃЌОнNa
2CO
3------------2HClЁЂNaHCO
3------------HCl
mol
mol
mol
mol
ЙЪД№АИЮЊЃКЂйЃОЂкЃОЂлЃЛ
BЃЎгыбЮЫсЭъШЋЗДгІЪБЃЌОнNa
2CO
3------------CO
2ЁЂNaHCO
3------------CO
2mol
mol
mol
mol
ЙЪД№АИЮЊЃКЂлЃОЂкЃОЂйЃЛ
ЃЈ2ЃЉНЋag Na
2CO
3КЭNaHCO
3ЕФЛьКЯЮяГфЗжМгШШЃЌОн2NaHCO
3Na
2CO
3+CO
2Ёќ+H
2OЁїm
168 62
m ag-bg
m=
ЃЌдђNa
2CO
3ЕФжЪСПЗжБ№ЮЊЃКag-m=ag-
ЃЌ
дђNa
2CO
3ЕФжЪСПЗжЪ§ЮЊЃКЃЈ1-
ЃЉЁС100%ЃЌЙЪД№АИЮЊЃКЃЈ1-
ЃЉЁС100%ЃЛ
ЃЈ3ЃЉИљОнЬтвтжЊЃЌдкЗДгІжаPbO
2ЕУЕчзгзЊЛЏЮЊЮЊPbЃЈNO
3ЃЉ
2ЃЌЫљвдЖўбѕЛЏЧІЪЧбѕЛЏМСЃЌдђЖўМлУЬЪЇЕчзгзїЛЙдМСЃЌШмвКГЪКьЩЋЃЌЫЕУїЖўМлУЬРызгБЛбѕЛЏЩњГЩИпУЬЫсИљРызгЃЌЫљвдЗДгІЕФРызгЗНГЬЪНЮЊ 2Mn
2++4H
++5PbO
2=5Pb
2++2MnO
4-+2H
2OЃЛ
ЙЪД№АИЮЊЃК2Mn
2++4H
++5PbO
2=5Pb
2++2MnO
4-+2H
2OЃЛ
ЃЈ4ЃЉвбжЊЃК
Ђй3FeЃЈsЃЉ+2O
2ЃЈgЃЉ?Fe
3O
4ЃЈsЃЉЁїH
1=-1118.4KJ/molЃЛ
Ђк2H
2ЃЈgЃЉ+O
2ЃЈgЃЉ?2H
2OЃЈgЃЉЁїH
1=-483.8KJ/molЃЛ
Ђл2H
2ЃЈgЃЉ+OЃЈgЃЉ?2H
2OЃЈlЃЉЁїH
1=-571.8KJ/molЃЛ
ИљОнИЧЫЙЖЈТЩПЩжЊЃКЂй-ЂкЁС2ЕУЃК3FeЃЈsЃЉ+4H
2OЃЈgЃЉ?Fe
3O
4ЃЈsЃЉ+4H
2ЃЈgЃЉ
ЁїH=ЁїH
1-2ЁїH
2=-1118.4+483.8ЁС2=-150.8KJ/molЃЛ
ЙЪД№АИЮЊЃК-150.8KJ/molЃЛ
ЃЈ5ЃЉ2.24LЃЈБъПіЯТЃЉCO
2ЦјЬхЕФЮяжЪЕФСПЮЊ
=0.1molЃЌ
ШєжЛЗЂЩњЃКCO
2+2KOH=K
2CO
3+H
2OЃЌдђЩњГЩ0.1molЕФK
2CO
3ЃЌЦфжЪСП=0.1molЁС138g/mol=13.8gЃЌ
ШєжЛЗЂЩњЃКCO
2+KOH=KHCO
3ЃЌдђЩњГЩ0.1molЕФKHCO
3ЃЌЦфжЪСП=0.1molЁС100g/mol=10gЃЌ
гЩгк13.8gЃО11.9gЃО10.0gЃЌЫљвдЕУЕНЕФАзЩЋЙЬЬхЪЧK
2CO
3КЭKHCO
3ЕФЛьКЯЮяЃЛ
ЩшАзЩЋЙЬЬхжаK
2CO
3 x molЃЌKHCO
3 y molЃЌ
ИљОнЬМдзгЪиКуЃЌгаЃКx mol+y mol=0.1 molЃЌ
гЩЖўепжЪСППЩжЊЃК138g?mol
-1ЁСx mol+100 g?mol
-1ЁСy mol=11.9g
СЊСЂЗНГЬЃЌНтЕУx=0.05mol y=0.05mol
дШмвКжаKOHЮяжЪЕФСПЮЊ 2xmol+ymol=2ЁС0.05mol+0.05mol=0.15molЃЌЫљгУKOHШмвКЮяжЪЕФСПХЈЖШЮЊ
=0.3mol?L
-1ЃЌ
ЙЪД№АИЮЊЃК0.3ЃЎ
ЕуЦРЃКБОЬтПМВщNa2CO3КЭNaHCO3аджЪЕФвьЭЌЃЌИЧЫЙЖЈТЩЕФгІгУЃЌИљОнЗНГЬЪНЕФМЦЫуЕШЃЌжївЊПМВщЗДгІЕФЖЈСПЙиЯЕМЦЫуКЭБШНЯЃЌжаЕШФбЖШЃЎ



 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ