
��������Ʒ�ڹ�ũҵ������Ӧ�÷dz��㷺���ش��������⣺

��1����̬��ԭ�Ӻ�����ӵ��˶�״̬��_______�֣���Щ���ӵĵ�������״��______�֣���ԭ�ӵ���Χ�����Ų�ʽΪ______________��
��2��NaHF2(��������)�����Ʒ�����NaHF2��������������������______________����HF2-��Ϊ�ȵ�����ķ�����______________��(��һ��)��
��3��N2F2(������ϩ)�����У���ԭ�ӵ��ӻ��������Ϊ_____________������N2F2���ܵĽṹʽ______��
��4��������������(��ͼ1)���۵�Ϊ-58�棬�е�Ϊ126��129�棬������____________���壮
��5�������Ƶľ����ṹ��ͼ2��ʾ������������=0.555pm��
��Ca2+��F-����λ���ֱ�Ϊ_______��_____��
����ʽ��ʾ�����ƾ�����ܶ�______________g•cm-3(���ؼ�������)��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꼪��ʡ��ԭ�и�һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����( )
A ����ӵ�ÿһ����ײ���ܹ�������ѧ��Ӧ
B ��Ӧ���������Ӻ���Ч��ײ�������࣬��Ӧ��������
C �ܹ�������Ч��ײ�ķ��ӽ��������
D ��������Ի�ѧ��Ӧ������Ӱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ�߶�5�µ��ж���ѧ�Ծ��������棩 ���ͣ�ѡ����
���в����ܴ��ʵ��Ŀ�ĵ���
A��������������������Һ�����������������Br��
B����CuSO4��Һ��ȥ�ɵ�ʯ�ͱ���ʳ��ˮ��Ӧ���ɵ���Ȳ�����е�����
C����2mol/L�������3mol/L��������������
D�������������������ƵĴ���Һ���Ⱥ����������ͨ�����Ը��������Һ�Լ�����ϩ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�캣��ʡ������ǰѺ�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��ҵ�Ͽ���ʳ�κ�ʯ��ʯΪ��Ҫԭ�ϣ�����ͬ�ķ������ɴ����ش��������⣺
��1��¬����������ʳ�Ρ�ʯ��ʯ��Ũ���ᡢ��̿Ϊԭ�ϣ��ڸ����½������գ��ٽ�ȡ���ᾧ���Ƶô��
��ʳ�κ�Ũ���ᷴӦ�Ļ�ѧ����ʽΪ�� __________________________________��
�������ƺͽ�̿��ʯ��ʯ��Ӧ�Ļ�ѧ����ʽΪ��__________________________ (��֪����������������������������ֻ��һ��)��
��2������Ĺ�������ͼ��ʾ���õ���̼�����ƾ��������ɴ��
��ͼ�е��м����C��___________��(�ѧʽ����ͬ)D��___________��
��װ�����з�����Ӧ�Ļ�ѧ����ʽΪ___________________��
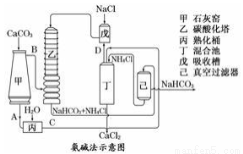
��3�������Ƽ�Ƕ���ĸĽ������ŵ��dz��˸������Ȼ�刺������������_______________��
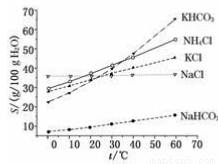
��4��������Ϊ̼�������̼�����ƵĻ�ѧ�������ƣ���Ҳ���ð�����Ȼ��غ�ʯ��ʯ��Ϊԭ����̼��ء�������ͼ���ܽ��(S)���¶ȱ仯���ߣ�����˵���Ƿ���У�_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�캣��ʡ������ǰѺ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʽΪ C5H8O2���л������NaHCO3��Һ��Ӧ�������壬���������������ͬ���칹��(�����������칹)��
A��8 �� B��10 �� C��11 �� D��12 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������и߶��µ��Ĵ��¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ�������
A��Ǧ�����س��ʱ��������Ӧ��PbSO4+2H2O-2e-�TPbO2+4H++SO42-
B�����Խ�����KMnO4����H2O2��2MnO4-+5H2O2 +6H+�T2Mn2++5O2��+8H2O
C����Ba(OH)2��Һ�еμ�ϡ���Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O
D�������ʵ�����MgCl2��Ba(OH)2��HCl��Һ��ϣ�Mg2++2OH-�TMg(OH)2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������и߶��µ��Ĵ��¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA��ʾ������������ֵ������˵����ȷ����( )
A��100mL0.1mol•L-1Na2SO4��Һ�У�����������0.03NA
B�����³�ѹ�£�32gO2-���������ӵ���ĿΪ17NA
C��1 molAl3+��ȫˮ���������������������ӵ���ĿΪNA
D����״���£�������ΪNA��N2��C2H4��������������ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ�߶��µڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�ɶ�����Ԫ�ع��ɵ����ӻ�������,һ�������Ӻ�һ�������ӵĺ��������֮��Ϊ20������˵������ȷ����
A��������һ��ֻ�����Ӽ���û�й��ۼ�
B�������������Ӻ������Ӹ�����һ�����
C������Ԫ��һ������ͬһ����Ҳ���ڵ�һ����
D�������������Ӱ뾶һ�����������Ӱ뾶
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ����ݿ���һ�и�һ���������ƻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͼʾ�仯Ϊ���ȷ�Ӧ����( )
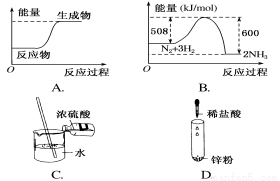
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com