
| ���� |
| ���� |
| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
| ���� |
| ���� |
| Ũ���� |
| ���� |
| Ũ���� |
| ���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
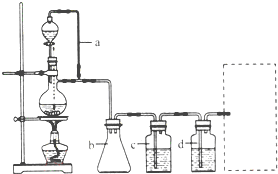 ��2011?���ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�
��2011?���ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�| һ������ |
| ||
| Ni���� |
| ||
| �� |
| ���� |
| ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?���ڶ�ģ��NaCl��NaClO�����������¿ɷ�����Ӧ��ClO-+Cl-+2H+=Cl2��+H2O��ijѧϰС�����о�����Һ����Ҫ�ɷ�ΪNaCl��NaClO���ı��������
��2012?���ڶ�ģ��NaCl��NaClO�����������¿ɷ�����Ӧ��ClO-+Cl-+2H+=Cl2��+H2O��ijѧϰС�����о�����Һ����Ҫ�ɷ�ΪNaCl��NaClO���ı��������| �����Լ� | Ԥ������ͽ��� |
| �Թ�A�м������� 1.0mol/LK�� ����Һ 1.0mol/LK�� ������Һ �Թ�B�м�1%Ʒ����Һ�� �Թ�C�мӢ� ����ʯ��ˮ ����ʯ��ˮ �� |
��A����Һ����ɫ��B����Һ����ɫ��C����Һ����ǣ�������Һ���ֱ��ʣ� �� ��A����Һ����ɫ��B����Һ����ɫ ���ޱ仯����C����Һ������ǣ��ޱ仯������ ����Һδ���� ��A����Һ����ɫ��B����Һ����ɫ ������Һδ���ʣ����ޱ仯����C����Һ������ǣ��ޱ仯������ ����Һδ���� �� ��A����Һ������ɫ���ޱ仯����B���� Һ����ɫ���ޱ仯����C����Һ��������� ��Һ��ȫ���� ��A����Һ������ɫ���ޱ仯����B���� ������Һ��ȫ���ʣ�Һ����ɫ���ޱ仯����C����Һ��������� ��Һ��ȫ���� |
| (2ab-5vc) |
| 50 |
| (2ab-5vc) |
| 50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| NaOH |
| �� |
 +NaOH
+NaOH| �Ҵ� |
| �� |
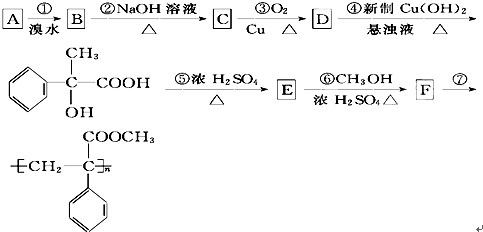






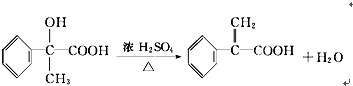

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com