
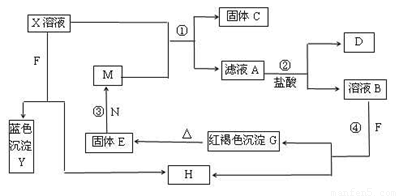
��֪XΪ��ѧ��ѧ�е�һ�ֳ������Σ�FΪ����ɫ���壻M��NΪ�����Ľ�����N��������������ͻ���ϣ�HΪ���嵥�ʣ�DΪ��ɫ���壬�ڿ����л���ֺ���ɫ�������ʵ�ת����ϵ����ͼ�����ַ�Ӧ��������ȥ����
��ش��������⣺
��1��X�Ļ�ѧʽΪ ��
��2���ڷ�Ӧ�٢ڢۢ��������û���Ӧ���� ������ţ���
��3����Ӧ�۵Ļ�ѧ����ʽΪ_________________________________��
��4��X��Һ��F��Ӧ���ܻ�ѧ����ʽΪ ��
��5��������ҺA�н������ӵķ����� ��
��6������100 mL��X����Һ�м���10 g������������M�ķ�ĩ����ֽ�����ˣ���ɵ�10.16g���塣����ҺA�����ʵ����ʵ���Ũ��Ϊ_________����������Һ������䣩
��1��Cu(NO3)2 ��2���٢�
��3��Fe2O3 + 2Al  Al2O3 +
2Fe
Al2O3 +
2Fe
��4��2Na2O2+2Cu(NO3)2+2H2O=2Cu(OH)2+4NaNO3+O2��
��5��ȡ������ҺA����NaOH��Һ��������ɫ������������ɻ���ɫ����ɺ��ɫ
��6��0.2 mol/L
��������
���������FΪ����ɫ���壬ӦΪNa2O2����ɫ����YΪCu��OH��2��N��������������ͻ���ϣ����ľ���Ӳ�Ⱥܴ����ֱ�����У�ӦΪAl2O3����NΪAl������������ˮ��Ӧ���ɵ�HΪO2��DΪ��ɫ���壬�ڿ����л���ֺ���ɫ����DΪNO��˵��XΪCu��NO3��2����A��D���ﷴӦ��֪A�к���Fe2+����MΪFe��CΪCu��GӦΪFe��OH��3��EΪFe2O3����1�������Ϸ�����֪XΪCu��NO3��2���ʴ�Ϊ��Cu��NO3��2����2���������з�Ӧת����ϵ��֪����Ӧ�٢۶��е��ʲμӺ����ɣ�Ϊ�û���Ӧ���ڢ�û�е������ɣ��϶������û���Ӧ���ʴ�Ϊ���٢ۣ���3����Ӧ�������ȷ�Ӧ����ѧ����ʽΪFe2O3
+ 2Al  Al2O3 + 2Fe ����4��Cu��NO3��2��Һ��Na2O2��Ӧ���ܻ�ѧ����ʽΪ2Na2O2+2Cu��NO3��2+2H2O=2Cu��OH��2+4NaNO3+O2�����ʴ�Ϊ��2Na2O2+2Cu��NO3��2+2H2O=2Cu��OH��2+4NaNO3+O2������5����ӦΪFe+Cu2+=Cu+Fe2+��n��Fe��=10/56=0.18mol����ȫ������Cu��n��Cu��=0.18mol��64g/mol=11.4mol������10.16g���壬˵��Fe����������ò��������㣬
Al2O3 + 2Fe ����4��Cu��NO3��2��Һ��Na2O2��Ӧ���ܻ�ѧ����ʽΪ2Na2O2+2Cu��NO3��2+2H2O=2Cu��OH��2+4NaNO3+O2�����ʴ�Ϊ��2Na2O2+2Cu��NO3��2+2H2O=2Cu��OH��2+4NaNO3+O2������5����ӦΪFe+Cu2+=Cu+Fe2+��n��Fe��=10/56=0.18mol����ȫ������Cu��n��Cu��=0.18mol��64g/mol=11.4mol������10.16g���壬˵��Fe����������ò��������㣬
Fe+Cu2+=Cu+Fe2+ ��m
1mol 8g
n 0.16g
n=0.02mol��c=0.02/0.1=0.2mol/L���ʴ�Ϊ��0.2 mol/L��
���㣺���⿼����������ƶϣ���Ŀ�Ѷ��еȣ������ע��������ʵ���ɫ����;��Ϊͻ�ƿڽ����ƶϣ�ע������ļ��㷽���İ��ա�


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
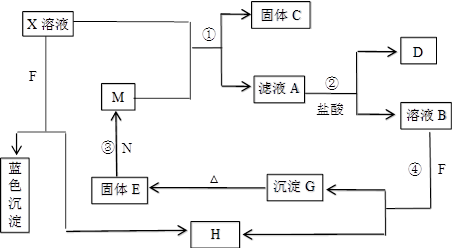
��8�֣���֪XΪ��ѧ��ѧ�е�һ�ֳ������Σ�FΪ����ɫ���壻M��NΪ�����Ľ�����N�����Ԫ�ص����Ӱ뾶�ǵ�������Ԫ�������Ӱ뾶��С�ġ�N��������������ͻ���ϣ����ľ���Ӳ�Ⱥܴ����ֱ�����У�HΪ���嵥�ʣ�DΪ��ɫ���壬�ڿ����л���ֺ���ɫ�������ʵ�ת����ϵ����ͼ�����ַ�Ӧ��������ȥ����
��ش��������⣺
��1��X�Ļ�ѧʽΪ ��F�ĵ���ʽΪ ��
��2����Ӧ�ڵ����ӷ���ʽΪ_______________________________________________��
��Ӧ�۵Ļ�ѧ����ʽΪ_______________________________________________��
��3������100 mL ��X����Һ�м���10 g��������M�ķ�ĩ����ֽ�����ˣ����
��10.16g����C������ҺA�����ʵ����ʵ���Ũ��Ϊ________________________��(�����������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ˮ��ѧ��һ��ѧ���������Ի�ѧ�Ծ� ���������� ���ͣ������
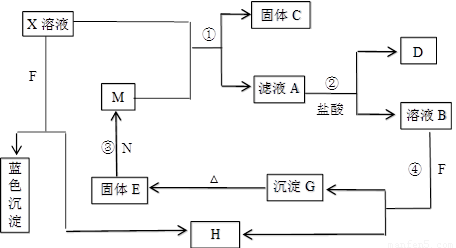
��8�֣���֪XΪ��ѧ��ѧ�е�һ�ֳ������Σ�FΪ����ɫ���壻M��NΪ�����Ľ�����N�����Ԫ�ص����Ӱ뾶�ǵ�������Ԫ�������Ӱ뾶��С�ġ�N��������������ͻ���ϣ����ľ���Ӳ�Ⱥܴ����ֱ�����У�HΪ���嵥�ʣ�DΪ��ɫ���壬�ڿ����л���ֺ���ɫ�������ʵ�ת����ϵ����ͼ�����ַ�Ӧ��������ȥ����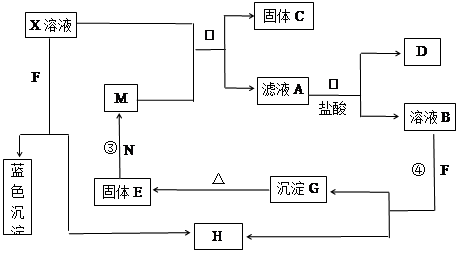
��ش��������⣺
��1��X�Ļ�ѧʽΪ ��F�ĵ���ʽΪ ��
��2����Ӧ�ڵ����ӷ���ʽΪ_______________________________________________��
��Ӧ�۵Ļ�ѧ����ʽΪ_______________________________________________��
��3������100 mL ��X����Һ�м���10 g��������M�ķ�ĩ����ֽ�����ˣ����
��10.16g����C������ҺA�����ʵ����ʵ���Ũ��Ϊ________________________��(�����������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ��һ��ѧ���������Ի�ѧ�Ծ��������棩 ���ͣ������
��8�֣���֪XΪ��ѧ��ѧ�е�һ�ֳ������Σ�FΪ����ɫ���壻M��NΪ�����Ľ�����N�����Ԫ�ص����Ӱ뾶�ǵ�������Ԫ�������Ӱ뾶��С�ġ�N��������������ͻ���ϣ����ľ���Ӳ�Ⱥܴ����ֱ�����У�HΪ���嵥�ʣ�DΪ��ɫ���壬�ڿ����л���ֺ���ɫ�������ʵ�ת����ϵ����ͼ�����ַ�Ӧ��������ȥ����
��ش��������⣺
��1��X�Ļ�ѧʽΪ ��F�ĵ���ʽΪ ��
��2����Ӧ�ڵ����ӷ���ʽΪ_______________________________________________��
��Ӧ�۵Ļ�ѧ����ʽΪ_______________________________________________��
��3������100 mL ��X����Һ�м���10 g��������M�ķ�ĩ����ֽ�����ˣ����
��10.16g����C������ҺA�����ʵ����ʵ���Ũ��Ϊ________________________��(�����������)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com