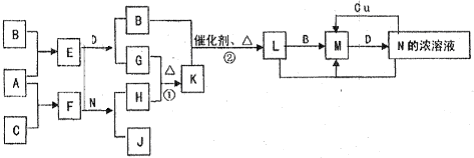
X��Y��Z��M���ɶ�����Ԫ����ɵĻ����X��Y��Z��������Ԫ�أ���Y��Z����������Ԫ�أ�Z����Һ�Լ��ԣ���ش��������⣺
��1��XΪ����ɫ��ĩ������DZˮͧ�Ĺ���������XΪ
��������
��������
��X�г���Ԫ���⣬����Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
��
��2��Y��Z��M�й�ʵ�����±���
| ʵ�� |
��Ҫʵ�鲽�輰ʵ������ |
| 1 |
��Y��Һ�м���ϡH2SO4����������ɫ��������ɫ�д̼�����ζ�����壬��������ʹƷ����Һ��ɫ�� |
| 2 |
��M��Һ�������μ�Z��Һ�����а�ɫ������������ܽ⣮ |
| 3 |
��Mϡ��Һ�еμ������ữ����������Һ��������ɫ������ |
��Y�������ӵĽṹ��ͼ��ʾ�����Կ�����SO
42-�е�һ��Oԭ�ӱ�Sԭ�����������д��Y��ϡH
2SO
4��Ӧ�����ӷ���ʽ��
S2O32-+2H+�TS��+SO2��+H2O
S2O32-+2H+�TS��+SO2��+H2O
��

��д����0.1mol Z����Һ��20mL 2.5mol?L
-1M����Һ��Ӧ�Ļ�ѧ����ʽ��
AlCl3+3NaOH=Al��OH��3 ��+3NaCl
AlCl3+3NaOH=Al��OH��3 ��+3NaCl
��
��3����ͼ��ʾ���������ʾ��������Һ��pH��������ɱ�ʾZn
2+��[Zn��OH��
4]
2-Ũ�ȵĶ���ֵ��lgc������ش��������⣺

��ZnCl
2��Һ�����Ե�ԭ����
���������ӷ���ʽ��ʾ��
����ZnCl
2��Һ�м�������Z��Һ����Ӧ�����ӷ���ʽΪ��
Zn2++4OH-�T[Zn��OH��4]2-
Zn2++4OH-�T[Zn��OH��4]2-
����ͼ�����ݿɼ��㳣����Zn��OH��
2��K
SPΪ
10-17
10-17
��



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�